Edit on 08/06/2024: At least one person has pointed out that, at one point, giving hypertensives at night were also thought to matter, a now disproven idea. Someone also mentioned how many times the clinical trial information was altered during the study. I added in a section at the end to discuss this.
There’s a really interesting phenomenon in the immunotherapy field that has been going on for what seems to be several years now, but was raised to me — a non-oncologist — via a viral Twitter thread of some work at ASCO25:
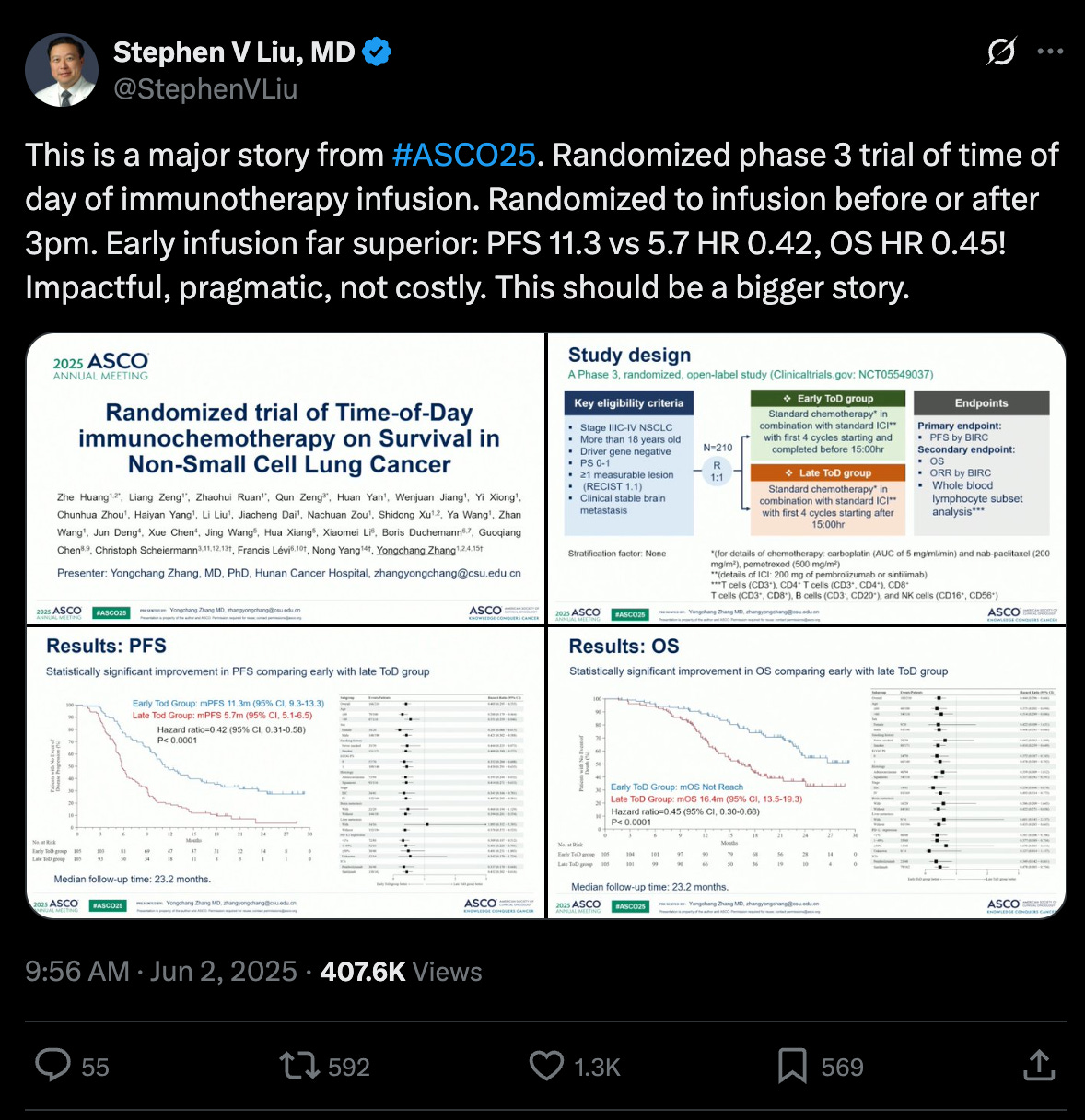
Translating the jargon: amongst the patients who received their immunotherapy infusion before 3pm (as opposed to after 3pm), their cancer stayed under control for longer (11.3 months vs. 5.7 months) and on median lived longer (at least 23.2 months versus 16.4 months). A near 2x~ improvement in the most important metrics doing something that is entirely risk-free and cost-free.
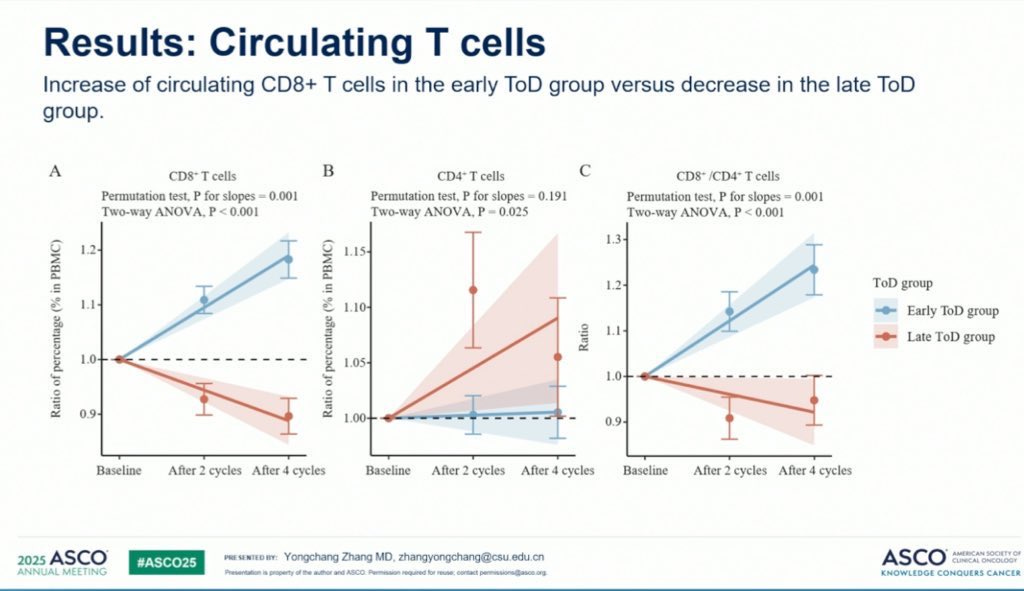
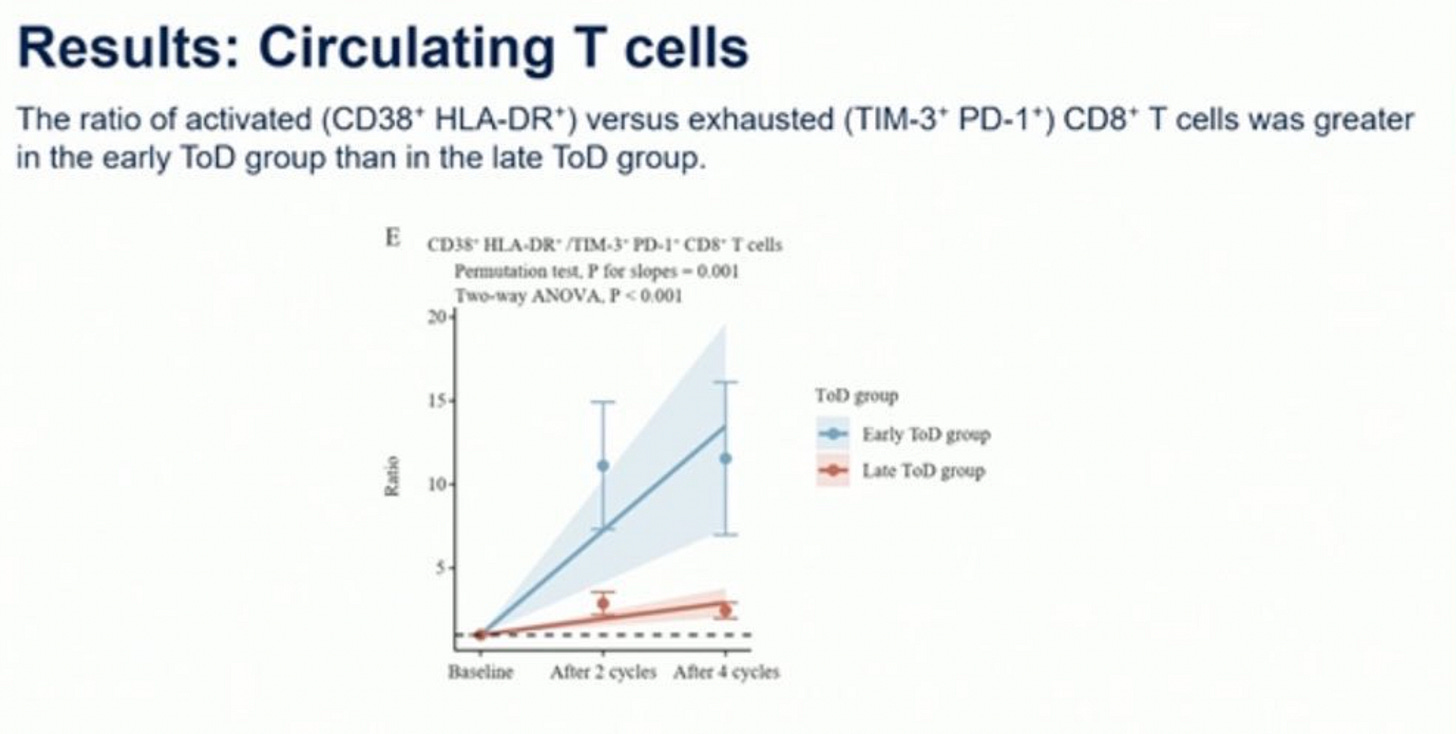
These two images shown in the comments of the post also demonstrate genuine changes in levels of circulating T-cells between the two groups:
Important context: the current standard of care for immunotherapy is not designed with timing in mind. You come in to get the injection when convenient for you or when there are free spots, there is no official recommendation to get it in the morning. But this study implies that we should potentially update our guidelines.
Weird, right? And if you have my relatively naive instincts, obviously wrong. Something must have been off in the study. After all, wasn’t there that one paper about how time-a-lab-test-is-taken is more predictive of patient survival than the test results themselves? The punchline? Sicker patients have strangely-timed emergency lab orders at 2AM, healthy patients have routine morning blood draws. Timing is hard to rely on!
But this paper was not a retrospective study of electronic health records, it was a randomized clinical trial, which is the gold standard. This means that we’ll be forced to immediately throw away our list of other obvious complaints against this paper. Yes, healthier patients may come in the morning more often, but randomization fixes that. Yes, patients with better support systems may come in the morning more often, but randomization fixes that. Yes, maybe morning nurses are fresher and more alert, but, again, randomization fixes that.
Okay. Well. Maybe there is something here. Caveats on this of course being a conference presentation without a corresponding, longer peer-reviewed paper, so we lack a lot of exact details on what exactly went on. Maybe the randomization used here is off for some reason, we’ll see once an official paper comes out.
But perhaps we should look beyond just this research. As it turns out, there is an astonishing amount of pre-existing literature on the immense benefits in giving patients immunotherapy earlier in the day, also known as ‘immunochronotherapy’. The exact time varies, but anytime before the evening seems to be good. Here’s one study that found, again, a massive improvement when giving immunotherapy before 11:30AM for advanced non-small cell lung cancer. And again for esophageal cancer, before 1pm. And again for melanoma, before 4:30.
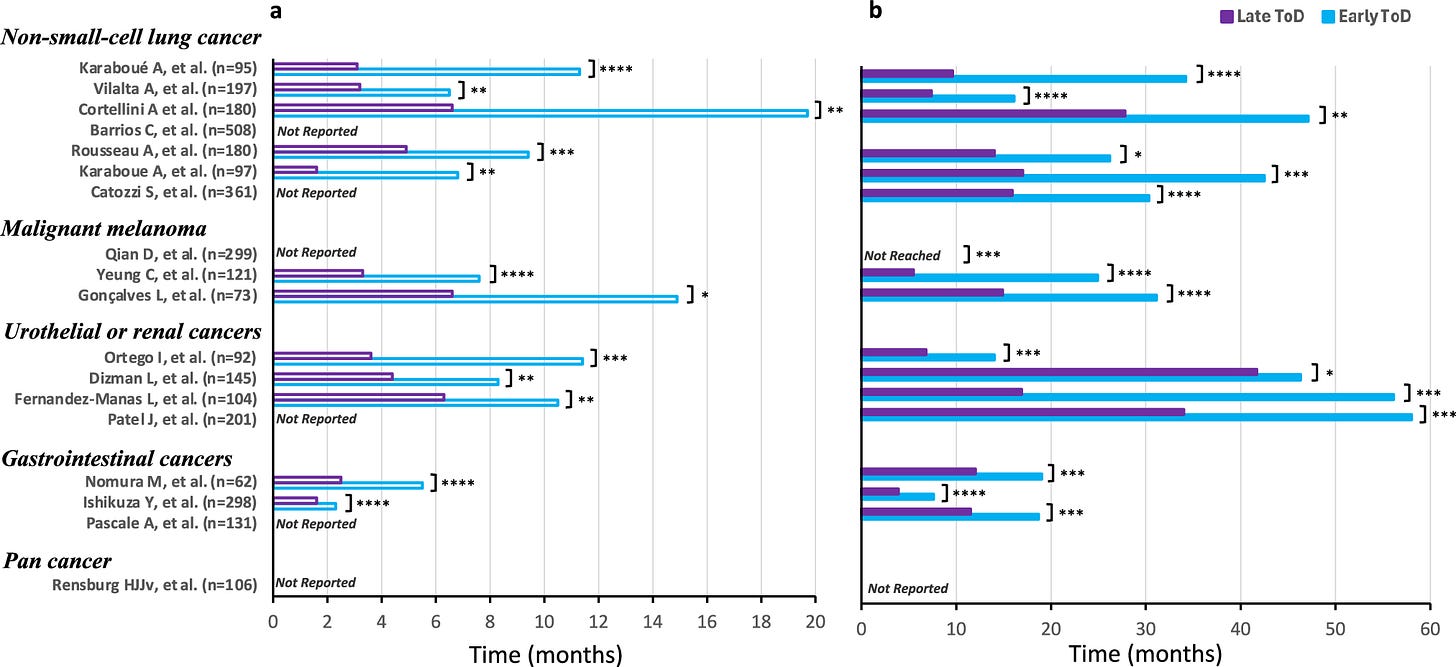
All of this culminated in a really incredible review paper that is really worth reading. It walked through 18 retrospective studies covering 3,250 patients, each of which studied the impact of immunotherapy injection time on patient outcome. And, once you compile them all together, there is a very dependable story being told across multiple types of cancer.
TLDR: early-in-the-day immunotherapy administration consistently leads to massive improvements in survival time, matching up quite well with the 2x results from the original Twitter post.
Keep in mind, these results have not only been shown for short-lived small molecules, but also long-lived proteins with half-lives on order of weeks that shouldn’t be affected by 24-hour cycles: pembrolizumab (Keytruda), nivolumab (Opdivo), and ipilimumab (Yervoy). Now, skepticism here would be justified given that these are all retrospective studies, and it’d be very easy for these to be confounded. But this evidence combined with the extremely similar results from the randomized clinical trial done I showed at the start of this essay should lead us towards at least suspecting that this is an honest-to-god free lunch.
What’s going on? Where is this coming from?
First, it’s worth reminding ourselves that the human body — and perhaps most complex life on Earth — exists on a schedule: the circadian rhythm. There exist 15-or-so ‘clock’ genes, like BMAL1, CLOCK, PER, and CRY, that oscillate with a rhythm. Not in structure or conformation, but in expression; the amount of them present in cells rises and falls over the course of the day. BMAL1 and CLOCK form a complex that drives the expression of PER and CRY. Once PER and CRY accumulate to a certain threshold, they feed back to inhibit BMAL1 and CLOCK, suppressing their own production. Over time, PER and CRY degrade, releasing the inhibition, and the cycle begins again.
One full loop takes just about 24~ hours, though there is some degree of individual variation.
So our cells have evolved to take advantage of these genes as an internal timestamp, a marker of where we are in the circadian rhythm. Some things occur early in the cycle, some things occur later, purely as a matter of convenience.
What’s the point of the cycle? One way to understand them is through an evolutionary lens, a way for the body to prepare for dependable environment cues.
For example, at the start of our circadian rhythm, we wake up. We crawl out of our safe cocoon — a private bed in modernity, or a predator-sheltered hole in ancient history — and start to engage in very risky behavior, immunologically speaking. Eating leftover food that may be contaminated, being scrapped by bacteria-covered rocks, holding dead animals to roast for dinner, and so on. But, as night comes, we retreat back to our private beds or holes, feasting on freshly cooked food, few interactions with unknown creatures, and little chance for injury as we wind down.
To anthropomorphize for a minute, millennia of evolution likely recognized this phenomena, and also noted that loading up an immune response is an unfortunately long process. A dendritic cell floating in the blood stream must first recognize + grab onto an antigen, then it needs to crawl into the lymphatic system, and then it hopes to bump into the few naive T-cell that recognizes that specific antigen. Then the adaptive immune response can kick off.
How could evolution optimize this process?
Well…if you didn’t have any priors on when new antigens would come through the door, you wouldn’t care when T cells decided to exit/enter the lymphatic system. When they exit, they are moving to new tissue. When they enter, they are actively looking for dendritic cells to bind to. Perfectly fine to do this randomly in the null case of uniform antigen exposure.
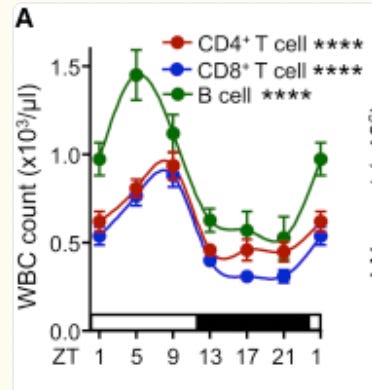
But! If you believe that antigen load is highest in the morning (which is something you can track via the clock genes), it would be smart to ensure that the lymphatic system is bloated with lymphocytes in the morning, removing their ability to migrate into the bloodstream. And according to one paper, that does empirically turn out to be the case in mouse models! Here’s a particularly useful graph:
The authors characterize the lymphocytes (T cells and B cells) of mice and find that it is highest during the resting period for mice (ZT 1-9), meaning that they are currently migrating throughout the body. Once the mice get closer to awakening (ZT10), circulating lymphocytes sharply drop, implying that they have moved themselves into the lymphatic system, awaiting for the morning antigens to arrive. Finally, the authors demonstrate that this entire process entirely depends on clock genes. If they are genetically edited out, bloodstream lymphocytes stay constant.
But this is just one immune-circadian tweak that evolution has made. Are there others?
How about prime T-cells such that they are more ‘willing’ to be activated by antigen-presenting dendritic cells at the start of the circadian rhythm? That exists. Perhaps improve the capacity for dendritic cells to migrate into the lymphatic system during points of low antigen exposure/during rest phases of the rhythm? That exists as well. Could we even allow the lymphatic system itself to become more permissible to entry? Technically, this was also a result from the prior paper, so this too exists. Maybe tilt the bodies hormonal signals such that such that immunosuppressive ones are minimized just before expected antigen exposure? Also exists!
Now, what is immunotherapy doing? In the common case of immune checkpoint blockades, it is simply allowing the immune system to more easily attack the cancer, since cancer typically chemically dampens their ability to do so. That’s all it does. It doesn’t provide new antigens, it doesn’t create new T-cell receptors, it doesn’t summon dendritic cells. Which means the effectiveness of that green light depends entirely on what the immune system is already doing at that moment.
Thus, we can propose a decent argument as to why immunotherapies seem to work best during the start of a circadian rhythm. The immune system, by evolutionary coincidence, is simply most prepared to begin their assault during that time.
But you may balk at this and say, “That would only make sense if immune checkpoint blockades had an extremely short half life that fit into this primed immune system period, but they don’t. To take an example, Pembrolizumab (Keytruda) has an extremely long half life of 27 days, is dosed every 3 weeks, and reaches steady-state blood levels at 19 weeks. How could it possibly be affected by initial infusion time?”.
Well, you’ve got me there! I am unsure what the answer could be. And as far as I can tell, so is everyone else, nobody has a clear, consistent answer to the question. But let’s take a stab at it.
Let’s pretend you have very early-stage cancer. The dendritic cells are in their normal cycle of desperately presenting tumor fragments to T cells, the T-cells rightfully getting upset, activating themselves, and going off to hunt the cancer. But cancer simply shuts them down by expressing an immune blocker protein: PD-L1. In response, the T-cell mostly shuts down, wanders back to the lymphatic system, and gets a little bit more ‘exhausted’. It believes that it activated itself for no reason, and thus will require a much higher bar for doing anything else in the future. The more times this occurs, the more exhausted the T-cell becomes, the more unwilling to ever activate again. In the limit, it will simply kill itself. Hence why you need immunotherapy to revitalize these cells!
Now let’s assume you received the immune checkpoint blocker Pembrolizumab at one of the best possible times: 7:30am in the morning, when the most T-cells are in your lymphatic system. Those get activated by the dendritic cells and are now finally able to attack the cancer, the checkpoint blocker preventing them from shutting down. Cancer is being killed! What advantages are you potentially privy to now as a result of the morning dose?
Of course all the ones we talked about earlier:
A greater number of T-cells are in the lymphatic system, so more opportunity to prevent exhaustion.
Dendritic cells are more aggressively collecting cancer antigens, so more opportunity for T cells to be activated.
The lymphatic system is more permissible to dendritic cell entry, allowing more interactions between dendritic cells and T-cells.
And so on.
But, all of this would also eventually happen if you had an evening injection. If we squint, the only downside an evening injection would have is that the highest concentration of the drug (at the moment of injection) does not have access to all of these advantages. But given the clearance rate of Pembrolizumab, only 1.25% of it would have dropped in the 12 hours from the evening injection → following morning. So the morning injection upside entirely stands on this +1.25% drug concentration bump. Either we are missing something, or the sum total of the initial 12-hour-long immune advantages are so high that +1.25% is extremely significant.
Perhaps the second take is genuinely true and answers the story entirely. Lots of immunologically useful things are going on in the morning, each contributing a little bit. As is often the case in biology, there is no singular causal factor for why early-morning immunotherapy seems to help so much, just many small things.
But let’s veer off into speculation. Maybe we are missing something?
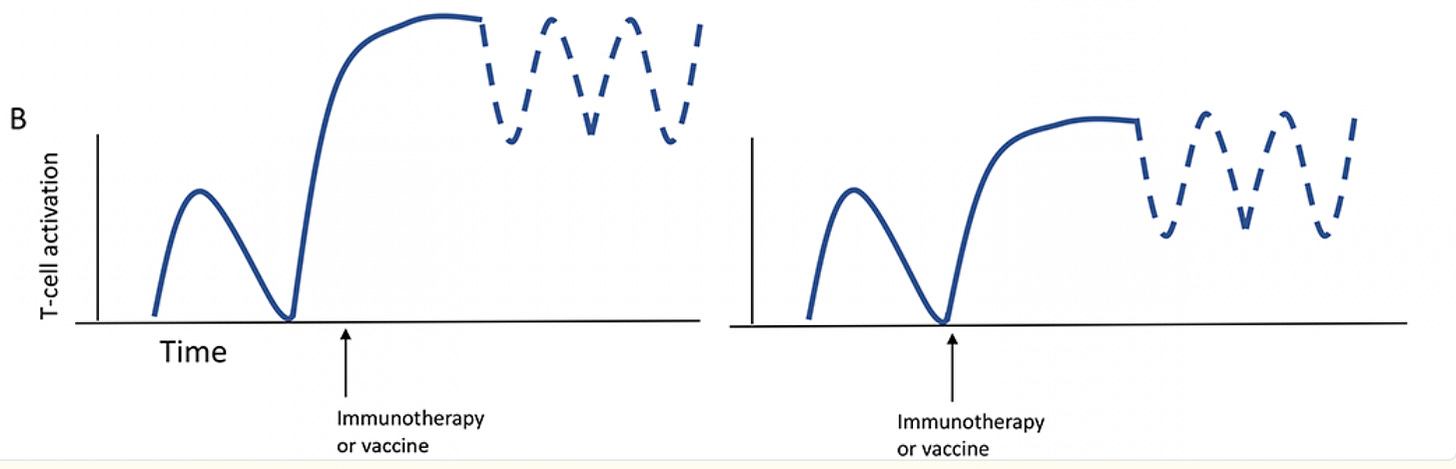
Perhaps we’re being overoptimistic on this idea of ‘steady state circulating antibodies’ being useful for T-cell activation. Maybe the first immediate dose of immunotherapy is the primary part that functionally matters for further T-cell activation. This idea was put forwards, albeit only theoretically, in a graphic from this paper.
Which is to say: each wave of activation of T-cells may set as a soft ‘ceiling’ of maximum immune response, even if the drug continues to circulate. So you’d ideally want the first ceiling to be as high as possible, which implies that a morning injection would be best! Is this true? Well, we do know that the clinical impact of the first immunotherapy injection is strongly tied to long term outcomes, and, accordingly, the timing of that first immunotherapy injection seems to matter the most. This same latter paper also says this:
…it appears that challenging the immune system with an antibody at a specific time of day not only changes the quantity but also the quality of the response so that the immune system, once stimulated at the “wrong” time, may not be able to respond anymore to the same level and quality as an immune system challenged at the “right” time—just 12 h apart.
Hence, why we should suspect that there is something fundamentally special about the first wave of activation of T-cells.
Of course, many questions follow from this. What is the temporal “window of imprintability” for T cells? Does that imply that early-activated T-cell clones dominate the final pool of T-cells? And what would mechanistically cause all of this? I don’t have the answer to any of these, and I suspect nobody does.
But again, maybe this is the wrong idea entirely, and there is no singular causal factor for these impressive time-of-day results. Maybe it is, once again, a bunch of small things — increased T-cell activation, but also stronger dendritic cell function and increased lymphatic vessel permissibility and many others — adding up to a strong signal.
For what it’s worth, we do know that this ‘early morning immunotherapy is useful’ phenomenon are also important for infectious disease vaccines, so it feels unlikely that this whole observation is entirely spurious. But vaccines mostly contain short-lived antigens and one-shot adjuvant signals, meaning they rely heavily on getting the initial priming window exactly right. That’s not the case for immunotherapy, so I suspect the benefits of morning injections in that context arise from a different mechanism—one that’s distinct from what makes morning timing valuable for vaccines.
We’ll see what the future holds. The phase 3 trial page that we talked about at the start is still ongoing and is currently the only randomized test of chronoimmunotherapy. But one more is getting kicking off for melanoma and there are calls for more to be run. Incredibly interesting subject, please reach out to me if you have any interesting light to shed here!
Edit on 08/06/2024
At least one person has mentioned that chronotherapy was also thought to matter for blood pressure medication, with rather convincing large retrospective studies, specifically the HYGIA trial. There was some mild mechanistic reason to suggest that circadian variation in sympathetic tone and cortisol levels could influence blood pressure regulation; going up when you sleep, potentially leading to more cardiac events. Thus, bedtime dosing of antihypertensives may prevent the ‘potentially harmful territory’ spike.
But multiple follow-up randomized studies, such as this and this largely disproved the whole concept. Given that, shouldn’t we be on guard for chronotherapy working in immunotherapy?
Well, yes! We should be on guard for everything, especially since our only major piece of evidence is a as-of-yet incomplete trial. But I’m personally erring on the side of the connection between the immune system and the circadian rhythm being much stronger than it is for other physiological functions, just given how large the lymphocyte concentrations in the bloodstream can shift from night to day. I’m also betting a little on the first wave of T-cell activation being particularly important, for reasons that are still not understood. Very open to being completely wrong though!
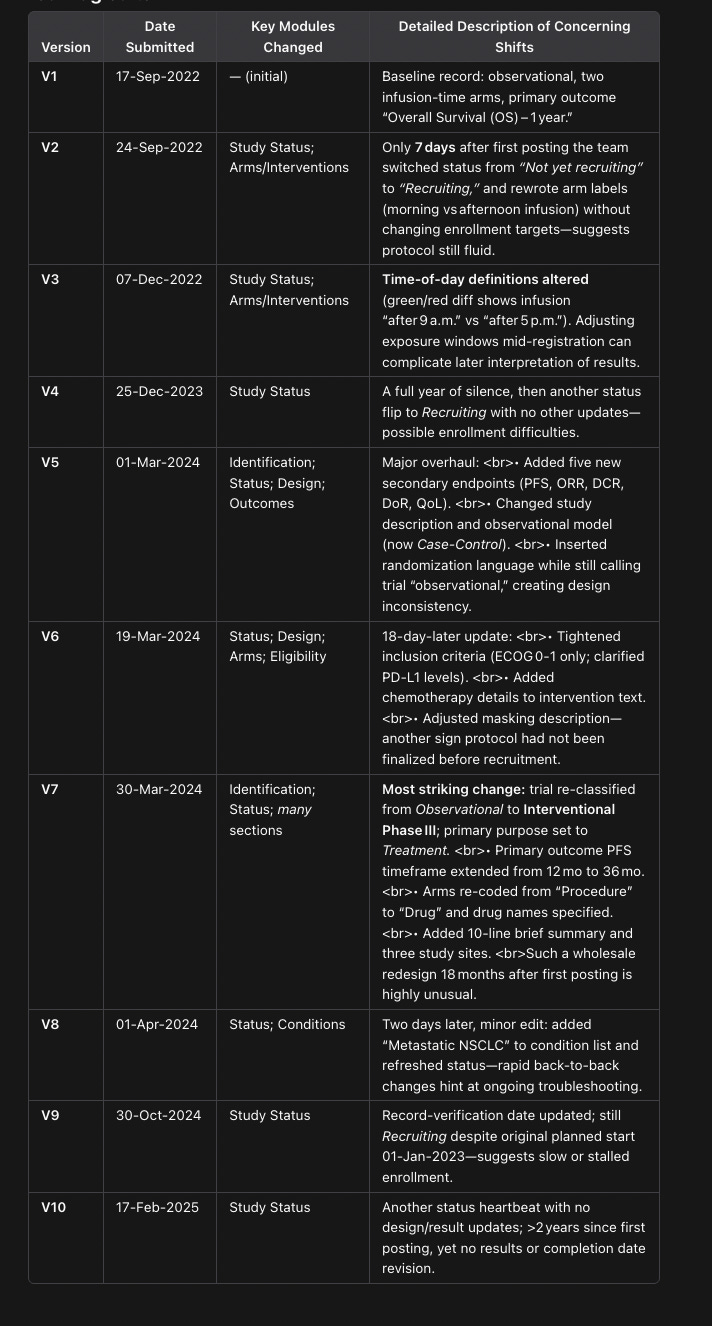
On a bigger note, someone else mentioned that the clinical trial methodology shifted midway across the 2 years of the study. I asked OpenAI’s Operator to create a table of the biggest changes made:
Which matches up with what the original poster says:
Which is concerning! And perhaps reason to discount the study entirely, mostly for the switch from interventional → observation → interventional. What’s up with that? The fact that it isn’t mentioned in the abstract either is also insane!
But I do consider the timing switches to be only mildly weird. Nobody has really figured out what is an optimal ‘early’ infusion, cut-off times can vary by 4-5 hours. Sure, they shouldn’t have amended it and stuck to one cut-off throughout, but the headline results seem strong enough that I’m not immediately worrying about them gradient-descending their way to a statistically significant result.
.png)