This paper is dedicated to the memory of a healthy person who started taking finasteride several years ago, “just” to improve his hair. Within a week, he developed severe neuropsychiatric symptoms that did not abate after stopping the drug. Treatment attempts by the best specialists did not help, and a few months later, he died by suicide.
Finasteride, a 5α-reductase inhibitor of testosterone conversion, was approved by the US Food and Drug Administration (FDA) in 1997 for treating androgenetic alopecia (AGA). The FDA recognized mental adverse reactions starting with depression in 2011 and then suicidality in 2022. The European Medicines Agency (EMA) acknowledged that finasteride can cause suicide in 2025. This might have been too late for some users of the medication. Could the neuropsychiatric risks from finasteride have been detected earlier?
In this paper, we will first review current evidence indicating increased risk for depression and/or suicidal behavior with the use of finasteride. We will then estimate the harms potentially caused by the delayed recognition of the neuropsychiatric risk associated with finasteride use. We will analyze the historical development and the causes for this delayed risk recognition. Finally, we will suggest policy recommendations for regulating and monitoring drug safety.
Current Evidence Indicating a Risk of Depression and Suicidality From Finasteride Use
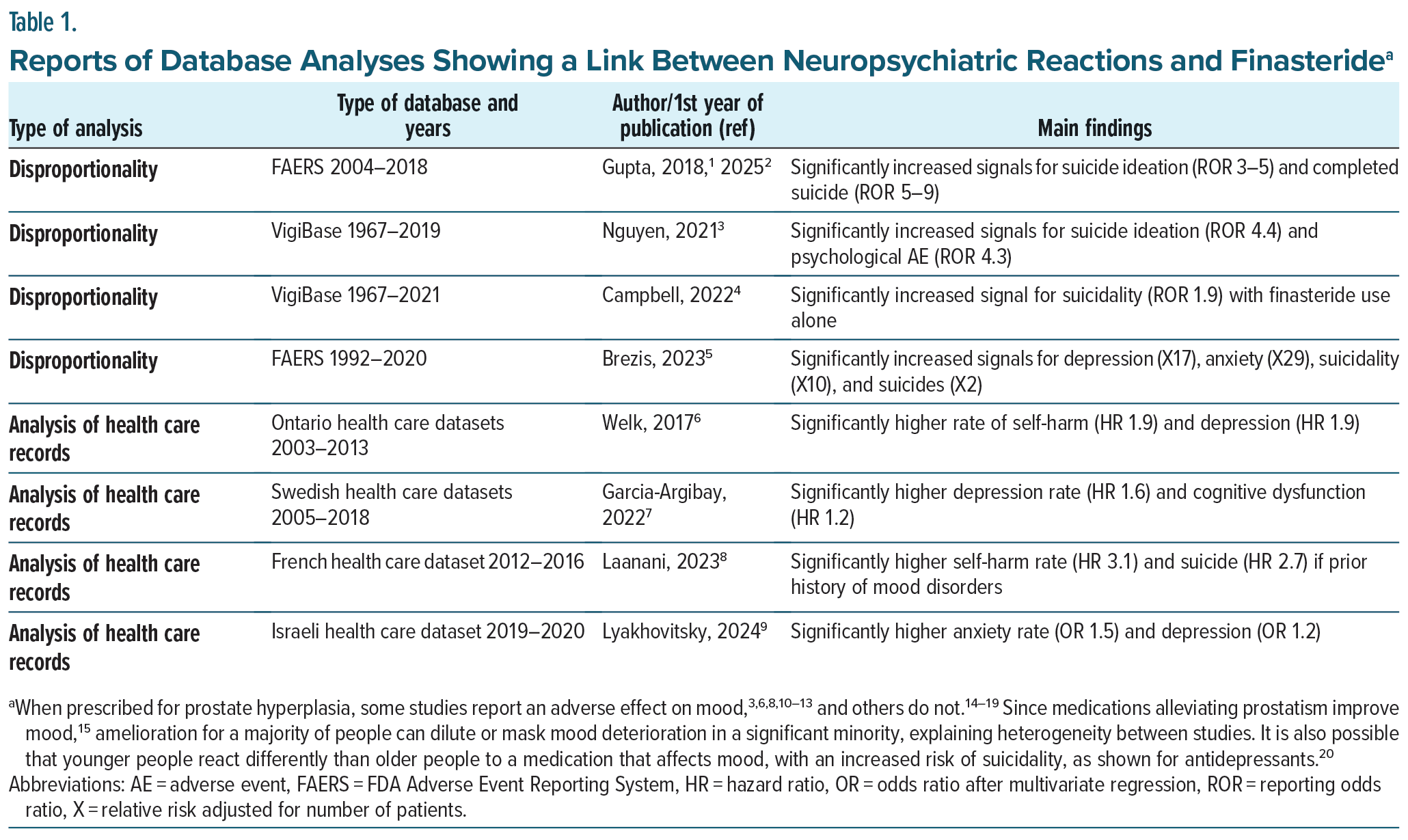
Proactive pharmacovigilance with analysis of databases uses two approaches. The first, disproportionality analysis, examines the number of adverse events reported to a database for finasteride in comparison to similar reports for other medications. The second approach uses data mining in large sets of patients’ records, comparing health outcomes for those treated with finasteride to controls with similar background confounders. Table 1 summarizes the studies in the last decade examining a potential link between neuropsychiatric reactions and finasteride exposure. When prescribed mainly for AGA, all reports suggest that finasteride can cause depression, anxiety, suicide ideation, and suicides. Assuming a null hypothesis (finasteride does not affect mood) and a 50% chance of 1 result against this hypothesis, the probability of getting all 8 studies concluding against the null hypothesis by chance is 0.58 = 0.0039.
As summarized in Table 1, analyses have confirmed that finasteride can cause serious mood disorders previously shown in several case series,21–26 a long-term follow-up of a large clinical trial,11 and a systematic review with meta-analysis,12,13 in concordance with a host of experimental studies,27,28 providing a biological explanation for these adverse effects of finasteride.
Biological Plausibility for Neuropsychiatric Effects of Finasteride
Animal and human studies have shown that finasteride, by inhibiting 5α-reductase, reduces the synthesis of neurosteroids, brain hormones that regulate mood.22,24,29 The reduction in neurosteroid levels, particularly allopregnanolone, is hypothesized to contribute to the neuropsychiatric side effects associated with finasteride use, such as depression, anxiety, and cognitive dysfunction.28,30 Often lasting long after medication discontinuation, neuropsychiatric reactions are sometimes severe enough to lead to suicide. Hippocampal neurogenesis, neuroinflammation, and genetic changes may mediate long-lasting effects of finasteride.27,31
Thus, experimental and epidemiologic studies show that finasteride can cause severe neuropsychiatric reactions, including depression, anxiety, and suicidality, even after the drug is discontinued, and the evidence for causality appears strong.
Potential Harms and Implications
Concerns about depression from finasteride were raised in several studies published as early as 2002. So, over 20 years worldwide, many may have endured depression and suicide. A relative increase in depression risk of 57% (averaging estimates in reports6,7,9) might increase suicide risk severalfold, as depression strongly predicts suicide.32,33 Extrapolating from the baseline prevalence of depression and suicidality in the population (see above, Table 2), exposure to finasteride of 4 million worldwide over 20 years might translate into hundreds of thousands who endured mood alterations up to depression with suicidality, while hundreds to thousands might have died by suicide.
Although speculative, these numbers indicate a potentially significant public health hazard. According to the precautionary principle,34,35 such a risk from a cosmetic medication suggests an unfavorable benefit-to- harm balance that justifies action to protect the public, and the burden of proving that the intervention is not harmful falls on manufacturers.35
Could the neuropsychiatric risks from finasteride have been detected earlier? We will now track the historical development leading to the recognition of these risks and the causes for this delayed risk recognition.
Historical Perspective
As early as 2002, 19 patients were reported to develop depression during finasteride treatment for AGA.36 They recovered after discontinuing the medication, and 2 agreed to a rechallenge, which was positive with depression relapse. In 2006, a prospective study on 128 patients showed a significant increase in depressive scores and concluded that finasteride should be prescribed cautiously for patients at high risk of depression.37 At this time, experimental studies had already shown an important mood-regulatory role for neuroactive steroids, which metabolism is altered by finasteride.38 This safety issue was then reviewed by the FDA.
Gross Underreporting of Depression and Suicides
In 2010, the FDA discussed including depression as a possible side effect of finasteride, but also noted that suicide ideation, attempts, and completed suicide were reported in numbers lower than expected.39 The actual degree of underreporting was not explicit as Merck kept the number of finasteride users confidential, with many areas censored on the FDA document, see, for example, in Figure 1: an entire section of text covered by a large gray-colored concealment.
It is difficult to imagine what data could justify hiding in a drug safety review. We now know that, already at this time, about 4.6 million patients were receiving finasteride worldwide.40 Based on the reported incidence of suicide and prevalence of suicide ideation in the general population,1,3,4 the expected numbers in a general population of this size treated with finasteride are calculated and shown in Table 2. The right-side column presents the actual numbers reported to the FDA Adverse Event Reporting System (FAERS), potentially related to finasteride usage. By 2011, only 18 suicides had been reported, while 6,440–12,880 were expected for 10 to 20 years of observation in a population of 4.6 million (and this figure is an underestimate, see footnote in Table 2). By 2024, a total of 320 suicides had been reported, while 19,320 were expected during 30 years of observation. For suicide ideation, expected numbers were 414,000, but only 31 and 1,062 were reported in 2011 and 2024, respectively.
There are multiple causes for this gross underreporting, including the fact that after completed suicide, the patient cannot report; the family may be unaware that the patient used finasteride; and the family and the physician may be unaware of a potential link between finasteride exposure and suicide. Similarly, for suicide ideation, the patient and physician may be unaware of a potential link to finasteride use. Awareness of underreporting demanded a different approach in pharmacovigilance beyond passive collection with individual analysis of single reports—in which the manufacturer repeatedly argued that causality between the outcome and finasteride exposure could not be established, was unlikely, or more likely due to premorbidity.
Why the Long Delay in Determining Suicidality Risk From Finasteride?
Clinical trials are designed to determine efficacy and are inadequate to test safety.41 Especially with accelerated medication approval, postmarketing surveillance needed to develop new tools,42,43 such as disproportionality analysis44 and data mining in healthcare records.45 These tools were quickly applied, for instance, to evaluate suicidality from weight loss medications.46–48 Why the long delay before determining suicidality risks from finasteride? Why were new tools for postmarketing surveillance applied (in the studies shown in Table 1), well over 10 years since initial reports suggested depression from the drug?36,37 Because of this delay, in 2019, post-finasteride syndrome was still thought of as a nocebo effect where patients suffer from delusions related to media coverage,49 even though adverse events reporting is not usually artificially stimulated,50 suicide has not been reported with the nocebo effect,51 and in the case of finasteride, increased awareness of a possible effect on mood appears to have uncovered a real problem.5
In a disproportionality analysis of FAERS data recently reported by Gupta et al,2 signals for suicidal ideations were reported in individuals taking finasteride only after 2013. This could reflect underreporting by clinicians and patients who were unaware of the possible link between finasteride and psychiatric risk (before 2011, when depression was first mentioned in the drug leaflet). However, the finding and its reporting took a decade to occur: the signal was buried in the FAERS data in 2013, waiting for a disproportionality analysis to uncover it. Still, it was discovered and published only in 2025 by a group independent from the manufacturer and the regulator. Thus, the signal could have been detected a decade earlier had the manufacturer and the regulator chosen to look for it.
As discussed above, in 2010, the FDA discussed including depression as a possible side effect of finasteride, but also noted that suicide ideation, attempts, and completed suicide were reported in numbers far lower than expected. Awareness of underreporting demanded pharmacovigilance research, such as that performed by Gupta, 15 years later. The FDA could and should have requested disproportionality analyses, but it did not. The manufacturer was aware of underreporting but dismissed the problem.
The Manufacturer’s Silence
Despite suspicion before 2011 leading the FDA to include depression as a finasteride side effect, not one of the studies presented in Table 1 was performed by Merck or requested by the regulator. The manufacturer’s failure to perform disproportionality analysis on adverse event reporting systems is remarkable since Merck itself scientifically examined the value of this tool, concluding in 2006 that52: “[It] demonstrate[s] sufficient sensitivity and specificity to be considered for use as an adjunct to conventional signal detection methods.” The company’s omission to perform data mining in health plan records is also surprising given that Merck has invested, since 2013, millions of dollars to get hands-on, real-world patient databases.53 These omissions contradict Merck’s claims on its website: “The safety of patients treated with our medicines is our top priority”54 and what Organon, another finasteride manufacturer, recently said: “Nothing is more important to Organon than the safety of our medicines and the people who use them.”55
It is also possible that studies were performed but not published. It is likely that, if performed, these studies would have detected worrisome signals as 8 others had in Table 1. It is also possible that Merck would dismiss the findings, as it had steadfastly denied that Vioxx increased the risk of myocardial infarction shown in its own studies.56
The omission or hiding of studies could relate to a backlash effect of litigation after the Vioxx fiasco, to stifle manufacturers’ incentives to monitor and research products’ risks.57 The industry’s commercial interest and loyalty to stockholders have often been shown to override concerns about drug safety.56,58,59 A classic role-playing exercise given to business school scholars showed that they often would fight a regulator-imposed ban on a medication suspected of deadly toxicity.60 Furthermore, for cost-effective research and development, the industry’s priority is naturally given to innovative drugs rather than to surveillance studies of old ones, especially with shorter patent life.
According to the legal doctrine of preemption, companies only have to meet FDA’s regulation, which would supersede state laws. This state of affairs emphasizes the important role of the regulator.
The FDA’s Ambivalent and Problematic Position
Regulators have exhibited difficulties in coping with the challenge of postmarketing surveillance. In 2010, the FDA should have requested that Merck perform the analytical studies shown in Table 1, as suggested in its guidance in 2005.61 Perhaps it did, but did not disclose this request to the public. It should have also pointed at the challenge of detecting an increase in suicides, even with big databases. Power analysis shows that to establish a doubling in baseline yearly suicide incidence rate (from 0.00014 to 0.00028, at α=.05, power 80%), the population followed should be over 7 million, and to detect a 10% increase (from 0.00014 to 0.00015), it needs a population of over 1 billion. Yet, looking at Table 2, such increases in suicide rates translate into over 12,000 or 1,200 deaths, respectively, after 20 years worldwide—a high toll that is preventable by greater attention to depression and suicide ideation as forecasting indicators of suicides. The FDA apparently also failed this task.
In 2017, the Post-Finasteride Syndrome Foundation submitted a petition to the FDA to remove finasteride from the market due to the risk of depression and suicidal ideation. In 2022, the FDA responded that it agreed to include suicidal ideation/behavior as an adverse reaction but not as a warning. It is unclear what made the agency take 5 years to generate this response, without requesting or performing any new study. Since the EMA had already included suicidal ideation as a warning in 2017, the FDA could have earlier adopted a similar decision based on the same evidence.
Nearly 20 years ago, at a dramatic congressional hearing following the Vioxx fiasco, FDA’s Dr. Graham warned that the agency was incapable of protecting the US from another drug safety crisis.62 He explained that superiors frequently criticized him and others in its Office for Drug Safety for bringing up disturbing findings about the adverse effects of FDA-approved drugs.62 The agency’s independence is in doubt since an increasing part of its budget is paid by the industry.63,64 In a marked example of biased decision-making, an FDA panel voted for a recommendation to allow Merck to bring Vioxx back into the market in 2005, after its toxicity was proven. It turned out that many members of this FDA panel had ties with manufacturers. Lack of transparency undermines public trust in the FDA.65 From a recent trial on the suicide of a finasteride user,66 it appears that already in 2010, FDA’s experts had recommended adding “suicidal thoughts and behavior” to the label. The advice was rejected by the agency without disclosing the internal discussion and the rationale for the final decision. Transparency is crucial when facing inherent conflicts between innovation and safety, progress and public health.
The FDA guides manufacturers to conduct pharmacovigilance.61 It seems, however, that the agency does not often request such studies with a strict timeline and careful enforcement, partly because understaffing faces an increasing overload of handling new medications at faster turnover.*
Considering Both the Patient’s Autonomy and Societal Costs
Drug safety should be discussed in the context of efficacy. Finasteride has been shown to be efficacious in treating AGA, although, according to a systematic review,67 most trials were small, short-term, and conducted by the industry with moderate quality of evidence and likely publication bias. A recent Bayesian network meta-analysis appears to challenge its usefulness in the long run.68 Yet, many young people like this medicine to enhance their appearance,69 often getting it over the internet without a physician’s prescription.70
An informed consent form has been suggested to help preserve a patient’s right to get a self-enhancement drug while being aware of its potentially severe side effects. Genuine shared decision-making is, however, unlikely to happen, as most physicians themselves are still unaware of the neuropsychiatric reactions to finasteride.71 Furthermore, the societal costs of these adverse effects are prohibitively high: the average direct and indirect costs (treatment and loss productivity) of depression are ∼$24,000 per patient per year.72 An increase in depression rate by 50% among finasteride users may translate worldwide into 200,000 people suffering from depression at a cost of 4.8 billion per year. This external cost, higher than the industry’s profit from the drug, makes it a bad deal for society.
Novelty and Limitations
This article provides a systematic review of postmarketing studies published over the last decade on the neuropsychiatric safety of finasteride. It specifically examines depression and suicidality using disproportionality analysis and healthcare records. To our knowledge, this is the first systematic review of postmarketing surveillance on finasteride. The article also provides estimates for the harms potentially caused by the delayed recognition of the neuropsychiatric risk associated with finasteride use. It analyzes the causes of this delayed risk recognition and provides recommendations for regulating and monitoring drug safety.
Significant limitations should be noted in this review. Evaluating harm from medications is challenging. Randomized clinical trials, designed to determine efficacy (intended effect), are inadequate for testing safety. Therefore, over the last two decades, epidemiologists have increasingly recommended using observational studies to detect and evaluate harms from medications (unintended effects).43,73–75 Pharmacoepidemiologic research on drug safety is evolving to include analysis of spontaneous reporting systems and health record databases.76 While using these tools, however, several challenges could temper the validity of a conclusion about a causal association between exposure to finasteride and neuropsychiatric reactions.
Although pharmacoepidemiologic studies are indispensable for detecting adverse drug reactions after marketing, they have several limitations: eg, indication bias, lack of data on co-medications, and incomplete information on psychiatric comorbidities. Incomplete and/or poor quality data on these potential co-founder variables could be especially challenging during the analysis of huge healthcare databases needed to demonstrate an increased rate of completed suicide among finasteride users (see power analysis above). Persistent neuropsychiatric reactions years after discontinuation of finasteride, or getting the drug over the internet, may lead to misclassification: drug users may be wrongly classified as controls according to the healthcare records.
Increased signals for suicidal behavior, detected on all 4 disproportionality analyses shown in Table 1, suggest a real safety issue but do not allow its quantification. The estimate for the number of suicides potentially caused by finasteride is speculative: Analysis of healthcare records from different countries (see Table 1) shows a significant increase in the rate of depression expected to be accompanied by a concomitant increase in suicide rate. A promising approach to estimate the magnitude of the problem would be a systematic recording of medication history for all suicides determined at a coroner’s or a medical examiner’s office, preferably using a cross-check of health care records. Comparing the rate of suicide in people exposed to finasteride to the rate of suicide in a control population might allow an estimate of the number of suicides caused by finasteride.77
The studies summarized in Table 1 address the effects of finasteride prescribed mainly for AGA. Could the psychological distress from hair loss cause the neuropsychiatric reactions attributed to finasteride? A recent systematic review and meta-analysis of the mental health impact associated with AGA found no association with depressive symptoms.78 By contrast, the studies shown in Table 1 reported increased rates of depression and/or suicidal behavior among finasteride users. In one study, among individuals with a history of mood disorders, finasteride was associated with an increased risk of suicidal behavior.8 It is, therefore, possible that prior mental dysfunction predisposes some people to severe neuropsychiatric reactions from the use of finasteride.
As indicated in a footnote to Table 1, there are conflicting results when finasteride is prescribed for prostate hyperplasia in older men. Some studies report an adverse effect on mood, and others do not. Contradictory evidence may have influenced the assessment and actions of regulatory agencies. Nevertheless, we did not find conflicting results in disproportionality analyses or healthcare records studies when finasteride was prescribed explicitly for AGA.
In conclusion, there has been a two-decade delay in recognizing the severe neuropsychiatric reactions to finasteride prescribed for AGA, allowing significant harm to occur from a cosmetic medicine. The delay derived from manufacturers’ failure to perform and publish simple postmarketing analytical studies, and the regulators’ failure to request them.
Implications for Policy
In accordance with the precautionary principle, the marketing of finasteride for alopecia should be put on hold until the industry can provide new evidence for its safety under whatever selection process—or until it can produce a molecule that does not cross the blood-brain barrier. Before approving a medication, regulators should require manufacturers to be bound to perform and disclose ongoing surveillance analytical studies, and that requirement needs to be enforced. We recommend systematic recording of medication history for all suicides determined at a coroner’s or a medical examiner’s office, preferably using a cross-check of healthcare records.
Materials and Methods
Over the last 5 years, an ongoing systematic review of the literature has been conducted to collect all clinical studies and pharmacovigilance research on finasteride’s safety. Repeated searches were performed, on average twice a year, using Google Scholar and Dimensions AI. The search strategy used combinations of the words Finasteride or 5α-reductase inhibitor with the words Adverse Effect, Suicide, Anxiety, Mood, Depression, Neurosteroids, Neuropsychiatric Reaction, Pharmacovigilance, Disproportionality Analysis, Data Mining, Drug Safety, or Post-marketing Surveillance. A Google Scholar alert was set to signal any new publication on finasteride. In addition, all literature reviews on finasteride were scanned for references to clinical studies on drug safety.
Preclinical and experimental studies, animal research, and systematic reviews were read but excluded from the analysis, leading to Table 1. For this table, we included only postmarketing studies on finasteride published over the last decade using either disproportionality analysis or data mining of healthcare records, looking specifically at depression and suicidality.
The internet was searched using Google for FDA documents about finasteride safety, and for publications by Merck or Organon about pharmacovigilance.
Power analysis for the sample sizes needed to detect an increase in the rate of suicide incidence was performed using the online calculator: https://clincalc.com/stats/samplesize.aspx.
For the number of finasteride users, we used the figure of 4.6 million indicated by a publication citing data from Merck for before 2010.40 The actual number of people having received finasteride over 10–20 years is likely much higher. People may have tried it for several days or weeks and then stop it, eg, because of sexual or mental adverse effects (some lasting after discontinuing the drug). Others may stop it after a few years of usage. If the average compliance over 5 years is around 50%, to maintain a figure of 4.6 million adherent users, the number exposed to the drug over 10–20 years would have to be 2–4 times higher. In its updated safety review of early 2025, the EMA mentions “an estimated exposure of around 270 million patient years for finasteride.” It is challenging to know the exact figure since many get the drug from the internet.79
Article Information
Published Online: September 22, 2025. https://doi.org/10.4088/JCP.25nr15862
© 2025 Physicians Postgraduate Press, Inc.
Submitted: March 11, 2025; accepted July 15, 2025.
To Cite: Brezis M. Failing public health again? Analytical review of depression and suicidality from finasteride. J Clin Psychiatry 2025;86(4):25nr15862.
Author Affiliations: Braun School of Public Health and Faculty of Medicine, Hadassah-Hebrew University Medical Center, Jerusalem, Israel.
Corresponding Author: Mayer Brezis, MD, MPH, Professor of Medicine (Emeritus) Hadassah Medical Center, Ein Kerem, POB 12000 Jerusalem, 91120, Israel ([email protected]).
Author Contributions: Conceptualization, data curation, investigation, and writing.
Relevant Financial Relationships: The author declares that he has no competing interests. He served as an expert witness on a trial about a person who died by suicide after using finasteride for alopecia. This case prompted a systematic review of the evidence about a potential causal link between this medication and neuropsychiatric reactions.
Funding/Support: None.
Clinical Points
- Finasteride, a cosmetic drug widely prescribed for hair loss, may cause depression and suicidality, even after the drug is discontinued.
- There has been a two-decade delay in realizing the extent and gravity of these neuropsychiatric reactions; hundreds of thousands may have endured depression, and many may have died by suicide.
- Policy recommendations for regulating and monitoring drug safety are suggested.
.png)