In addition to releasing the best T1D predictor in the world, we have published two academic papers this week on our website: a comprehensive essay on the ethics of embryo screening, and the validation paper for our cognitive ability predictor, CogPGT 1.0. The ethics paper explores why parents should be permitted to use polygenic scores to guide embryo selection, while our new validation paper establishes that substantial and robust within-family genetic prediction of cognitive ability is now feasible.
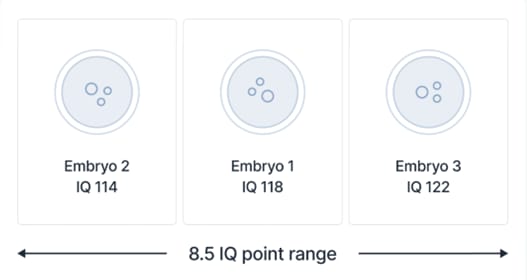
We think intelligence is a trait that many parents using IVF will wish to consider alongside a variety of health traits when prioritizing which embryo to transfer. Indeed, as we show below, intelligence is associated with a variety of life outcomes, including overall health and length of life. For this reason, while intelligence is quite different from disease traits like diabetes and breast cancer, it can also be seen as a trait that contributes to psychological and physical well-being (Ritchie, 2015).
For years, the predictive power of polygenic scores for cognitive ability has been limited. Our new paper presents a polygenic score (PGS) that achieves a substantial increase in accuracy, validated in two separate, independent cohorts. In the UK Biobank (UKB), CogPGT explains 16.4% of the variation in fluid intelligence among unrelated individuals, a significant leap over previous predictors. This was achieved through a novel approach using extensive phenotype engineering and deep learning-based imputation to maximize genetic signal from the available data.
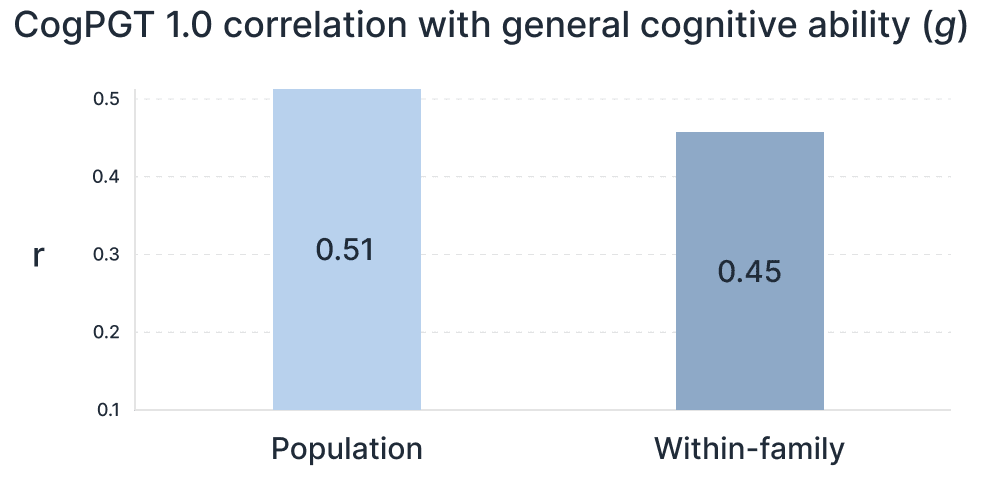
Crucially, the score maintains its power when making predictions within families—the only context that matters for embryo selection. Within sibling pairs, genetic differences arise purely from the random shuffle of parental genes. Our PGS shows only a slight reduction in this scenario, achieving a within-family correlation with latent general cognitive ability (‘g’) of 0.456 in UK Biobank adults and 0.448 when meta-analyzed with the adolescent ABCD cohort (Figure 2). This robust within-family performance demonstrates the score is capturing direct genetic effects, not just correlations related to family environment or population structure.
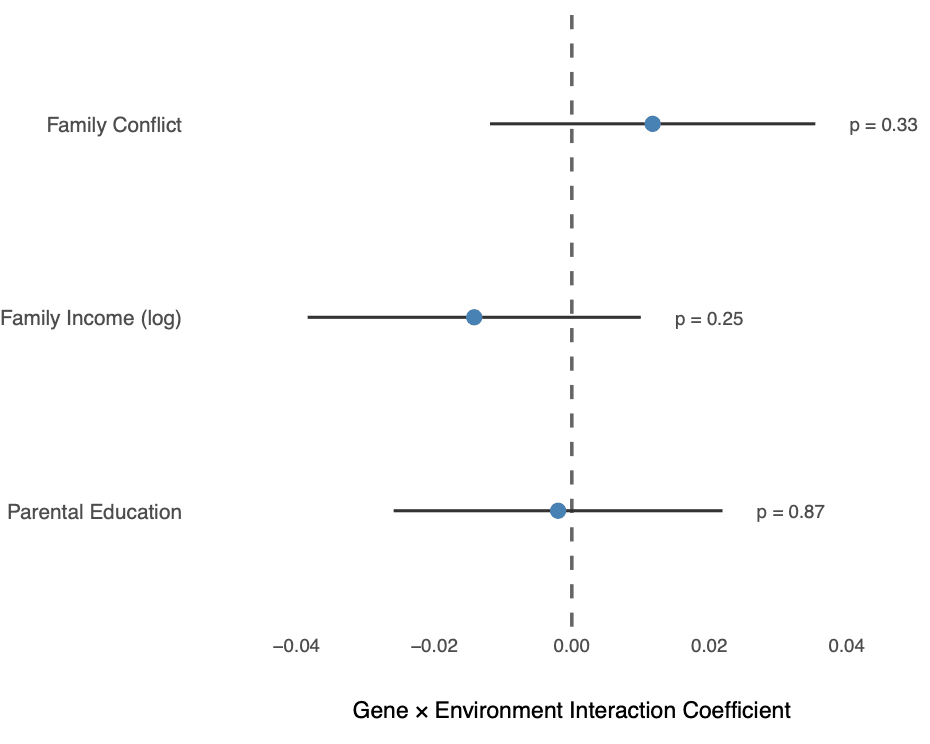
Not only is the score highly predictive even within families, but its effect is robust over different social contexts. We tested for gene-environment interactions and found no evidence that CogPGT’s effect on cognitive ability was altered by key environmental factors like family income, parental education, or family conflict (Figure 3). This suggests the score is capturing a stable genetic signal, providing parents with a reliable piece of information to factor into their decision-making, regardless of their socioeconomic background.
A higher genetic predisposition for cognitive ability is associated with better physical health (Arden et al, 2015). Our analysis shows these are not just correlations but likely reflect causal effects. We tested CogPGT 1.0 against 20 common diseases within UK Biobank families and found a broadly protective pattern, particularly for cardiometabolic conditions.
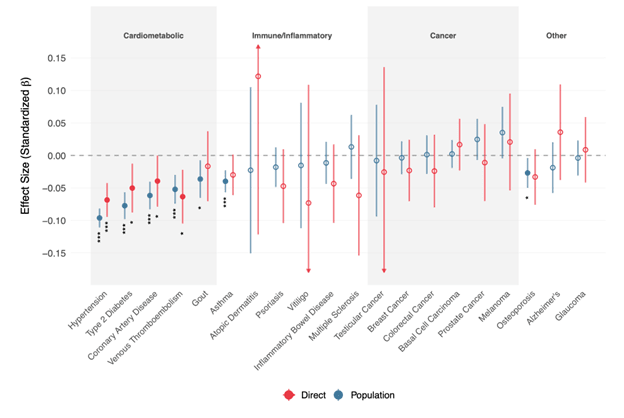
Even after accounting for shared family effects, a higher CogPGT score was associated with a lower risk for all five cardiometabolic diseases studied, including hypertension, type 2 diabetes, and coronary artery disease (Figure 4). This provides strong evidence that genetic factors influencing cognitive ability also play a causal role in physical health and disease prevention. For this reason, we think that some parents using IVF will wish to consider cognitive ability along with many standard health traits such as various cancers and autoimmune disorders.
In what follows, we begin by arguing that two widely accepted moral considerations support embryo screening for cognitive ability: parental autonomy and the welfare of future people. We then reply to common objections to embryo screening and conclude that parents using IVF have strong reasons to be permitted to make informed choices about which embryo to implant.
Autonomy is the bedrock of modern medical ethics. Governments and physicians respect parental autonomy by permitting them to make choices about themselves and their children.
There are many reasons we should treat parents as the default decision makers over which embryo to select for implantation. One reason is that parents tend to have better incentives to act in the interests of their potential children than the state would. Since parents will bear the burdens and benefits of raising their children, they are likely to think carefully about how they can benefit their children most. In the context of IVF, this may include choosing an embryo – from a set of embryos – that embodies the profile of risks that parents think will make their children best off.
Another reason for deferring to parental choice is that parents tend to be in a better position than states or insurance companies to express their values through their reproductive choices. Some may disagree with the choices people make in selecting a partner, a gamete donor, or an embryo. People value different traits in partners and children. But the principle of autonomy says that, except in unusual cases, we should recognize the rights of parents to make their own choices in the marriage market and the fertility clinic.
This autonomy matters most when parents can access meaningful information. Our previous work demonstrates that polygenic scores now provide such information in the context of screening for common diseases. In our new validation paper, we show that the same logic holds for intelligence.
The second reason to honor parents’ desire to use polygenic scores to choose which embryo to implant is that parents have reasons to want to minimize disease and select traits they believe will promote their children’s welfare. Parents already spend a fortune on education and health care after their children are born in order to promote health and well-being. Some parents also wish to influence the traits of their children before they are born.
Julian Savulescu (2001) argues that we ought to select an embryo that would result in a child with the best chance of the best life. Savulescu asks us to consider the case of a woman who can delay pregnancy by a few months to avoid her child developing a severe abnormality. Although the child that results from waiting a few months would be a different child, and thus the benefit is conferred onto a hypothetical person, it is still intuitively obvious that she has good moral reason to wait before becoming pregnant. By analogy, if a couple could reliably and affordably test their embryos for Down syndrome, or for rheumatoid arthritis or type 1 diabetes, it would be morally dubious for them to spend their money gambling in Las Vegas rather than obtaining information about the genetic risk profile of their embryos.
When thinking about which among a set of embryos to implant, most parents would not deliberately choose the embryo with the highest disease burden. This is because healthier people will spend less of their time and money being sick, getting surgeries, and worrying about dying prematurely. Healthier people also tend to have positive externalities for other people with whom they share the planet. People with a healthier immune system are less likely to transmit infectious diseases, and those with fewer illnesses will impose fewer social costs on families and communities.
A similar case could be made for intelligence. Smarter people excel in science and other intellectual arenas. But they also tend to contribute more to public goods, which affects the quality of the institutions that we share (Kanyama 2014). The reason they exhibit more cooperative behavior in competitive situations like public goods games – situations where there are immediate personal gains from defection, but larger gains in the future from cooperation – seems to derive from the fact that intelligence is positively correlated with patience, long-term thinking, and self-control (Jones 2015).
This isn’t merely theoretical: in our UKB validation sample of 4,642 sibling pairs, our polygenic score for intelligence predicted not only cognitive test performance but also educational attainment, family income and occupational status within families–outcomes that shape life trajectories (see Figure 5).
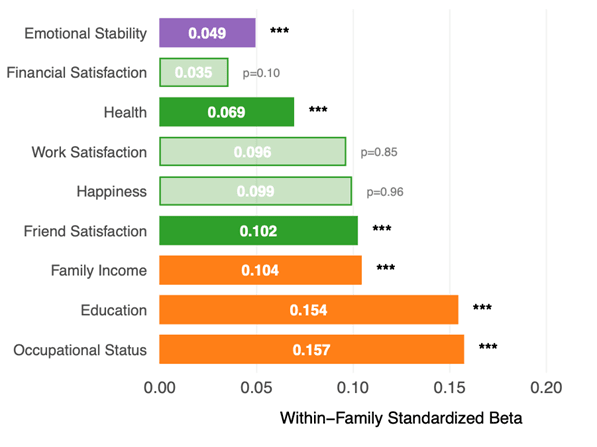
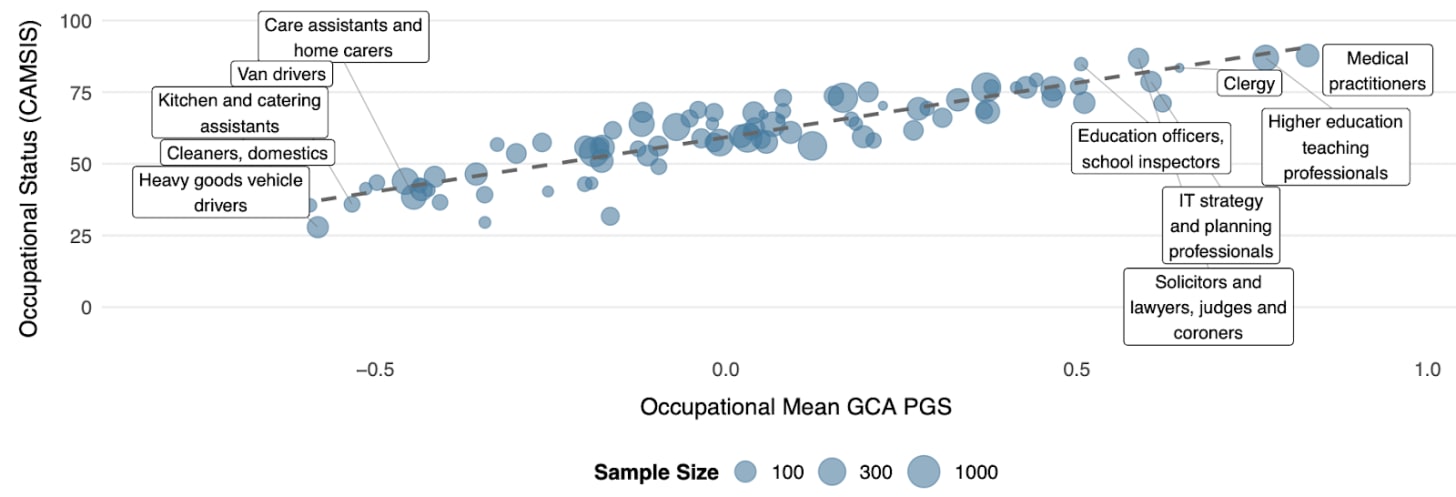
The link to occupational roles is particularly striking. As Figure 6 illustrates, the average CogPGT score aligns systematically with the cognitive demands of different professions and closely tracks occupational status. Occupations like medical practitioners, lawyers, university lecturers and IT professionals rank among the highest, while manual occupations rank among the lowest.
These considerations give us moral reasons to allow parents to select against disease and in favor of intelligence (Anomaly and Jones 2020).
Many people seem to equate ethics with whatever is widely believed to be right at a particular moment in time. But ethical beliefs can change quickly, especially given how elite opinion drives human attitudes and behavior (Henrich, 2015). For this reason, we think it is worth understanding what people actually believe about embryo screening. But we also think it is worth asking what people are likely to believe when they have more information about embryo screening.
According to a recent survey, about three quarters of Americans already favor allowing parents to select embryos in order to reduce physical and mental disorders (Furrer et al, 2024). According to another survey, about half of young Americans think it’s ethically defensible to select embryos for cognitive ability (Meyer et al, 2023). A solid majority of Singaporeans say they would select embryos for both health traits and cognitive ability, and agree that doing so is ethically acceptable (Haining et al, 2024).
While survey evidence already suggests many people consider PES morally justifiable, we think even more support will emerge in the near future.
After all, new technologies often provoke skepticism. When IVF was first introduced in the 1970s, many Americans condemned it as “unnatural.” But the tide turned as soon as people understood that IVF was safe and effective. Once most doctors and other influential people embraced it, ordinary people did too. We think the same will happen with PES, even if it requires a preference cascade for elites to publicly support it. Either way, the objection that there is widespread opposition to embryo screening is false.
A common worry is that PES and related technologies might increase genetic inequalities so much that it will undermine social harmony. There are several responses to this worry.
First, there are already large genetic inequalities between people, and these are growing as people increasingly have children with other people similar to their own abilities, including intelligence. Unless we force everyone to marry people who are very different from themselves in order to produce a distribution of children with more equal traits, inequalities are bound to emerge along several dimensions. And unless we force everyone to use the most powerful forms of genetic technology, we cannot prevent some genetic inequalities from emerging from the reproductive choices parents make.
Second, at least for now, the most important way to stack the genetic deck for your children is to choose your partner (or sperm or egg donor) carefully. While it is true that embryo screening can significantly reduce the risk of diseases such as schizophrenia and diabetes, there are limits on how much it can be used to boost traits like intelligence and height.
These limits are partly a function of how many embryos a couple can create, and how much genetic variation emerges when sperm and egg combine their DNA to form an embryo. In the absence of extreme genetic luck, embryo screening is not going to give otherwise ordinary parents the ability to create geniuses and giants in the way multiplex gene editing might.
Moreover, as mentioned above, the worries some people have about cognitive inequalities that may arise through embryo screening should consider both the personal and social benefits associated with having bright people in the population.
Another kind of inequality could occur because PES works a bit better for some groups than others. The “ethnic portability” problem derives from the fact that there are genetic differences between ethnic groups, and these differences grow in proportion to how genetically distant an ancestral group is from a group from which polygenic scores are derived (Privé et al, 2022).
Polygenic scores derived from a Scandinavian population like Sweden, for example, will work less well for Arabs, and even more less well for sub-Saharan Africans or Australian aborigines, who are more genetically distant from Scandinavians. The problem is difficult to solve since polygenic scores require the creation of large biobanks that are accessible to researchers around the world.
Because of challenges associated with ethnic portability, some seem to think that it’s morally bad for anyone to use PRS to guide embryo screening before the scores work equally well for all groups. We disagree.
First, consider an analogy with medications. There is strong evidence that some medications aimed at treating hypertension are more effective in some ethnic groups than in others (Brewster and Seedat, 2013). Suppose, then, that a medication is more effective at treating hypertension in Africans than it is at treating hypertension in Asians. Should we prohibit Africans from accessing the medication until a drug of comparable efficacy and price is available for Asians? We don’t think this would be morally justified or politically feasible, unless we accept the principle that nobody should be allowed to use a medical resource until everyone has equal access to that resource, parents should be permitted to use polygenic risk scores to select which embryo they wish to implant.
Second, if we want to equalize access to polygenic scores in the long run, allowing people to use them now will send signals to governments and other entities that better data should be collected on ethnic groups that are currently underserved. Demand is the ultimate incentive for supply to emerge. Demand can come in the form of price signals (for companies to offer better PRSs for everyone) and political rewards (in the form of lobby groups and voting blocks punishing politicians who don’t allocate resources toward improving data collection in the service of PRSs).
Economists like to say that “there’s no free lunch,” and evolutionary biologists often point out that everything has a cost. In a sense, this is true: the heritable traits we have now must have passed through many different kinds of filters. Because evolution did not consciously search for the optimal ways of creating traits that help us survive, it had to rely on mutations, as well as permutations of the genetic code, that might have produced both benefits and costs. This is the problem of pleiotropy.
“Pleiotropy” occurs when genetic variants have multiple phenotypic effects. A dramatic example is the beta-globin HbS variant prevalent in parts of Africa and the Middle East: inheriting one copy offers protection against malaria, but two copies result in sickle-cell anemia. Knowing this causal relationship enables parents to use embryo screening to minimize the risk of severe genetic disease.
However, pleiotropy raises the question of whether selecting an embryo with genetic variants known to have beneficial effects—such as lower risks of heart disease or breast cancer—could also increase other unknown or unintended negative effects.
In response to this concern, we conducted a comprehensive empirical analysis to assess the extent of antagonistic pleiotropy – which occurs when a set of genetic variants have beneficial effects on one trait but harmful effects on another – and its implications for embryo screening. Specifically, we examined genetic correlations across 2,871 traits against 31 potential candidate traits that parents may wish to influence via embryo screening (these include traits like diabetes and height). After refining the set to remove redundant or ambiguous traits, we retained a final set of 1,117 phenotypes with clear beneficial or adverse orientations for analysis, leading to 34,627 total genetic correlations analysed. Out of these, 3,040 were statistically significant and just 37 (or 0.16%) were negative (i.e., implying potential antagonistic pleiotropy).
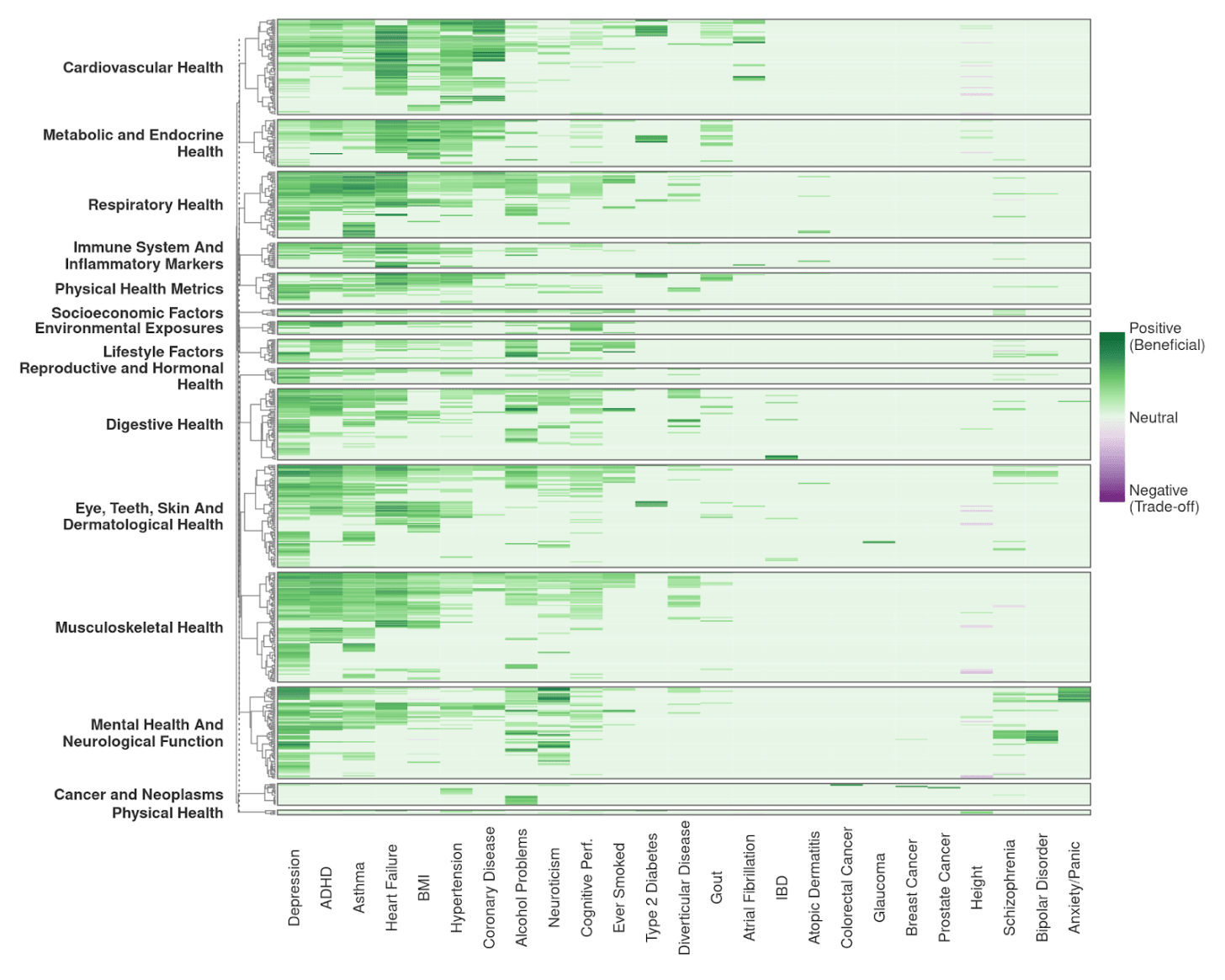
Figure 8 visually summarizes these genetic correlations, with green cells indicating neutral or beneficial genetic relationships and purple cells highlighting instances of antagonistic pleiotropy.
Several findings stand out:
First, most of the trait correlations that we observed are beneficial. This suggests that selecting embryos based on polygenic scores for commonly targeted health and cognitive traits is unlikely to systematically introduce unforeseen negative effects. To the contrary, selecting against a disease trait is likely to have downstream beneficial effects due to synergistic (positive) pleiotropy. Our empirical analysis, which confirms findings by a previous team (Widen et al 2022), effectively shifts the burden of proof onto those who use pleiotropy as an argument against embryo screening.
Second, antagonistic pleiotropy is rare and occurs in clearly defined clusters rather than being widespread across traits. The identified clusters correspond closely to well-documented biological pathways. For example, height is correlated with increased risk for heart problems due to larger atrial size.
This is not surprising, as it is well known that extremely tall people tend to suffer from knee injuries and cardiovascular stress as they get older. But this example illustrates another point: in many cases of antagonistic pleiotropy genes do not directly cause a bad outcome like arthritis in the knees, but instead make these outcomes more likely emerge from bodies that are much larger than the average human height. In fact, we think it is good for people to know this, so that they don’t just select for taller children without recognizing potential health risks as they move well above average.
Just as selecting against common diseases tends to produce positive rather than negative pleiotropy, so too does selecting in favor of intelligence. Figure 9 below (from our CogPGT validation paper) shows that intelligence appears to exhibit mostly positive pleiotropy.
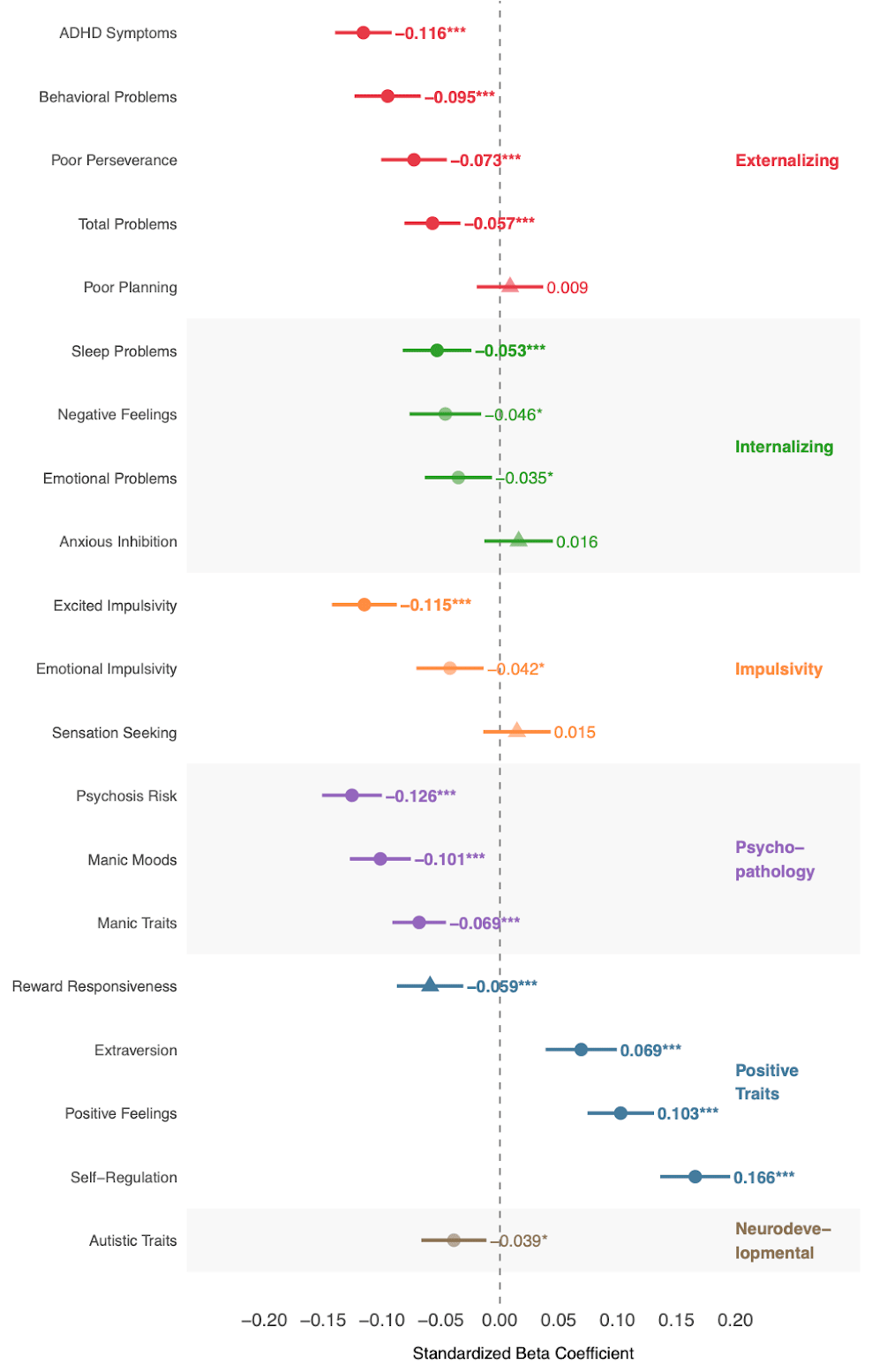
More specifically, a higher cognitive polygenic score robustly correlates with fewer psychotic-like symptoms, lower ADHD symptoms, reduced externalizing behaviors, fewer sleep problems, and increased positive affect. Particularly noteworthy, we observed no significant positive association between our cognitive polygenic score and autistic symptoms; indeed, the direction was weakly negative, contradicting earlier concerns derived from genetic correlation estimates. This research will need to be replicated, of course. And it is important to emphasize that the accuracy of estimates for pleiotropic effects depends on how accurately people in biobanks are phenotyped (that is, how accurately they are classified as having a particular disease or trait).
In sum, our analysis supports treating pleiotropy as a manageable part of a complex decision faced by parents who use polygenic scores to inform embryo screening. Health care providers have ethical reasons to present cases of antagonistic pleiotropy to prospective parents. But the rarity and clear biological grounding of antagonistic pleiotropy suggest that polygenic embryo screening, accompanied by appropriate genetic counseling, can have clear benefits to future children without introducing substantial risks of unintended harm.
Some people worry that if polygenic scores can effectively reduce the risk of disability and disease, those born with congenital challenges will be socially stigmatized, or will be discriminated against by employers, health insurance companies, or governments.
It’s worth stressing that polygenic embryo screening doesn’t eliminate disease or disability, but only changes the chances that an embryo will develop a condition. Nevertheless, if large numbers of parents use IVF and then test their embryos for single-gene disorders like Tay Sachs, chromosomal abnormalities like Down syndrome, and polygenic diseases like schizophrenia, they will reduce the rate of inherited disease in a population, rendering some conditions rare.
It is conceivable that those remaining with these diseases would be branded with social stigma or face discrimination.
One concern is that people born with higher risks of disease or abnormality will be blamed for their traits, or considered intrinsically flawed – biologically or morally inferior. This is indeed how Nazi leaders treated people with mental and physical disorders. They were not only stigmatized, but, in extreme cases, euthanized.
There are no guarantees that developed societies will promote norms of respect for those with congenital disease or disability. But in our own society, for example, parents using IVF have been selecting against monogenic diseases like Tay Sachs and Huntington for decades, and there seems to be no rise in intolerance or stigma toward those born with such conditions. We suspect the same will be true for polygenic diseases.
How we treat people with disease or disability depends on cultural norms more than technological developments. In a society that prizes tolerance, and promotes norms of respect rather than disdain for the disabled, they are less likely to be stigmatized and discriminated against.
Moreover, wealthy societies with robust public health infrastructure can afford to treat the disabled with more social and legal protections. Many cultures expose the weak and disabled to the elements when resources are scarce. The Inuit are especially famous for this. This gives us reasons to promote social institutions that help us avoid extreme resource scarcity, while also strongly supporting legal and social norms of toleration for the disabled.
Francis Galton coined the term “eugenics” to refer to “the science which deals with all the influences that improve and develop the inborn qualities of a race” (1883). By “race,” Galton meant a group of animals, in this case the human race. In the words of Leonard Darwin – past president of the eugenics society of England, and the fourth son of Charles Darwin – “eugenics is the study of heredity as it may be applied to the betterment, mental and physical, of the human race.
Galton’s and Darwin’s definitions of “eugenics” are silent about whether the state should play a role in guiding parental choices. They simply say that the science of heritability should guide our reproductive choices. Which practices should influence our reproductive choices – whether arranged marriages in India, courtship customs in Victorian England, libertine sexual norms in San Francisco, subsidized genetic testing in Dubai, or infanticide for deformed children in ancient Sparta – has been fiercely debated both before and after Galton coined the term.
Nevertheless, because of the atrocities committed by the National Socialists in the name of eugenics, many people now use “eugenics” as a kind of curse word to label and condemn anything that involves genetic explanations of behavior, or preferences by parents for certain genetically-influenced traits. We reject this way of framing the issue, since it erects taboos around important topics like behavioral genetics and the ethics of embryo screening. Taboos preclude clear thinking about how to use genetic information to achieve socially just outcomes (Buchanan et al, 2000).
People indirectly think about heritable traits in the dating and mating markets. They often do so implicitly in the form of what traits they’re attracted to – e.g. indicators of fertility or a low disease burden, such as smooth skin – and explicitly when they’re considering having children, especially in the context of IVF. Few people think there is anything morally wrong with wanting our children to be healthy or attractive, and it’s clear that we would not have survived as a species unless people chose their partners partly on the basis of genetically-mediated traits that gave them and their offspring advantages.
When gay or infertile couples select a sperm or egg donor, they rarely choose at random. Instead, they inquire about a prospective gamete donor’s health, achievements, personality, and so on. Relationships are more indirect ways of discovering this information, even if we don’t explicitly think about them in this way. And when people are ready to have children, they often say things like “I hope he has his mother’s smile,” or “let’s hope she doesn’t develop her father’s temper.”
We reject the term “eugenics” as a label for embryo screening, though we also recognize that many critics will call it “eugenics” with the intention of stigmatizing it. When accusations about “eugenics” are made, critics should at least specify what they mean by it, and why a particular instance of it is good or bad.
We reject the darkest forms of state-sponsored eugenics, such as forced sterilization and murder. But we think it is perfectly reasonable for parents to test their embryos for traits that promote human welfare and minimize unnecessary suffering. Branding such testing “eugenics” does nothing to honor past victims of unethical past policies, and it does a lot to obscure the important moral and political issues associated with embryo screening.
We’ve argued that parents should be free to screen their embryos using polygenic scores. We’ve also addressed common moral concerns scholars have expressed.
Our validation work demonstrates this is no longer hypothetical. Polygenic scores can now meaningfully distinguish between embryos for health traits and, critically, intelligence. Our comprehensive pleiotropy analysis suggests that concerns about hidden trade-offs are largely unfounded, in fact, our data indicates that screening for cognitive ability is associated with broadly positive psychological and health outcomes. We think that when negative pleiotropy exists, parents should be made aware of it to inform their choice of which embryo to implant.
While governments may play a role in minimizing information asymmetries or punishing deceptive advertising, we think the risk of government overreach in the reproductive realm exceeds risks associated with bad parental choices. Individual liberty constrained by social norms rather than government coercion should be the principle guiding the ethics of embryo screening, whether for complex diseases or for cognitive ability.
If you are interested in learning more, please take a look at our ethics article and our cognitive ability validation paper. Subscribe to our newsletter for more updates.
.png)



