Introduction
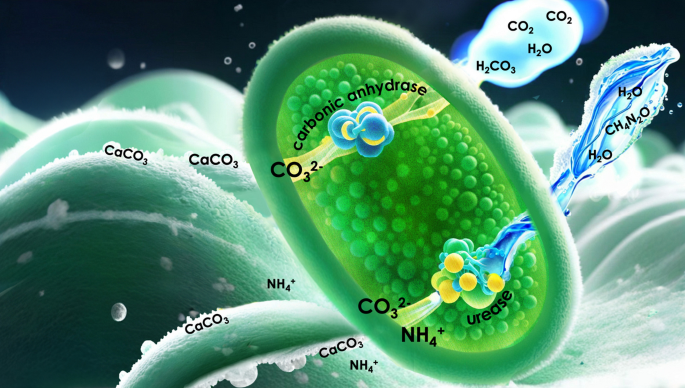
In this work, we aim to investigate a less explored microorganism in the traditional field of microbially induced calcite precipitation (MICP), which is dominated by ureolytic strains1,2,3,4. To understand the motivation behind this work, one should consider that the traditional MICP pathway involves the breakdown of urea (an organic fertilizer produced via the combination of liquid CO2 and liquid ammonia) by urease-producing microorganisms such as Sporosarcina spp. into CO32− and NH4+ (Eq. 1). The ammonium ion is an unwanted byproduct that must be completely removed as a trade-off in the otherwise beneficial MICP process, in which CaCO3 precipitate acts as a binder for building applications, commonly referred to as biocement (Eq. 2)5.
$$\text{CH}_4\text{N}_2\text{O} + 2\text{H}_2\text{O} \to 2\text{NH}_4{}^{+}\: + \text{CO}_3{}^{{2-}}$$
(1)
$$\text{Ca}^{2+}\:+\:\text{CO}_3{}^{2-} \leftrightarrow \text{CaCO}_3$$
(2)
In addition to traditional ureolytic MICP, among the different metabolic pathways that facilitate carbonate precipitation, the use of carbonic anhydrase (CA) is attracting interest. In recent works, microbes with genetic information for the CA enzyme have been mobilized in an attempt to eliminate ammonium, for example, in the work of6, in which Bacillus licheniformis was used in bioaugmentation protocols to improve clay structure. In this method, the applied MICP pathway emerged from a preliminary step in which hydrated aqueous CO2 formed H2CO3 upon reaction with water and subsequently ionized, yielding HCO3− and water (Eq. 3). The newly ionized carbonate anion then combined with free Ca2+ cations to form CaCO3 in one of its metastable phases, i.e., into the amorphous calcium carbonates (ACCs) vaterite or aragonite or to directly form calcite (Eq. 2). CA is known to serve as a nucleation site in this process6,7.
$$2\text{HCO}_3{}^{-}\:+\:2\text{OH}^{-}\:\leftrightarrow\: 2\text{CO}_3{}^{2-}\:+\: 2\text{H}_2\text{O}$$
(3)
We therefore focused on Bacillus megaterium, a species with genetic determinants for both the enzymes urease and carbonic anhydrase. These two pathways, in fact, can be activated at the same time synergistically8, but it is unclear whether selective activation can occur. Mineralization of gaseous CO2 by B. megaterium has been reported in9, and given increasing interest in the direct use of atmospheric CO2 in industrial processes, in this study, we suggest a framework for tracing the carbon source of precipitated CaCO3 and quantifying the fraction that originates from CO2 (Fig. 1).
Materials and methods
Bacillus megaterium is an aerobic gram-positive bacterium commonly present in soils10. It received its unique name from its dimensions: 4 μm long and diameter of 1.5 μm11. The interest in this taxon lies in its versatility; it has been applied for recombinant protein production12, soil bioremediation13, and microbially induced precipitation14. The enzyme urease catalyses the reaction, and one mole of urea is hydrolysed to ammonia and carbonic acid15. This leads to an increase in pH and the number of negative ions on the cell surface, which attract positively charged calcium ions, leading to CaCO3 precipitation. B. megaterium has the ability to exploit another pathway to induce carbonate precipitation10 via CA-mediated precipitation. An alkaline environment induces a reaction between calcium ions and HCO3− and ultimately leads to calcium carbonate precipitation. Nevertheless, environmental conditions, such as pH, temperature16 and the presence of ions like magnesium17,18, have a complex and significant effect on the precipitation of CaCO₃ polymorphs, influencing not only the crystallization process but also the morphology and stability of the resulting phases. Despite this, the selective activation of ureolytic metabolism as a mechanism for controlling CaCO₃ polymorphism remains less explored, warranting further investigation to better understand its role in microbial-induced calcium carbonate precipitation.
CaCO3 precipitation via the urease pathway
To confirm the urea hydrolysis pathway in MICP, a first round of growth experiments (U test) was performed in aerobic 100 mL flasks on the basis of conditions proposed by19. B. megaterium (DSM 32) was grown in 15 g/L yeast extract, 10 g/L peptone, and 10 g/L NaCl (growth medium) for 24 h. All the media components were obtained from Sigma Aldrich unless otherwise indicated. Subsequently, 4.5 mL of B. megaterium culture was inoculated (OD600 = 0.7) in 30 mL of media supplemented with urea and CaCl2. Both components were filtered through 0.22 μm filters (Sigma‒Aldrich) and added in equimolar proportions at 1.5 M. The flasks were incubated in triplicate for four days at 30 °C and 100 rpm. Daily feedings of the growth medium were performed, and pH was monitored at the end of each experiment together with precipitate yield, absorbance and electrical conductivity (see Supplementary Figure S1). One should consider herein that alkaline environments can be linked to the presence of peptone itself (10 g/L), which may influence the pH of the medium, or potentially magnesium, which is known to preferentially precipitate aragonite20. However, magnesium was not used in this study. Furthermore, abiotic control samples, including peptone, were tested in this work and yielded no precipitation, supporting the notion that the presence of peptone alone does not significantly impact the absorption or hydration of CO₂ in the culture medium. Data from these abiotic tests are provided in the Supplementary Figure S1. In CO₂-infused media, suboptimal pH conditions for CaCO₃ precipitation are expected due to acidification. While such environments are often considered unfavorable for biomineralization, recent studies show that B. megaterium retains urease activity and supports calcite formation even below neutral pH. More precisely21 observed measurable growth and precipitation at pH 6–7, and Manna et al. (2024)22 confirmed these findings across a broader acidic-to-alkaline range. These results indicate that CaCO3 precipitation cannot be excluded under mildly acidic, CO₂-rich conditions. Additionally, we postulate that peptone concentrations around 10 g/L are typically sufficient to support bacterial growth and promote ammonia production from urea (which was supplied at 90 g/L), thus contributing to medium alkalinization, as described by23. These starting hypotheses highlight how these factors—alkaline environment, peptone, and the absence of magnesium—contribute to the formation of CaCO₃ polymorphs via enzymatic pathways in the proposed experimental system.
CaCO3 precipitation via the carbonic anhydrase pathway
The second round of experiments (CA test) was designed to evaluate CaCO3 formation via the carbonic anhydrase pathway. For this purpose, 2.5 mL of B. megaterium grown for 24 h was inoculated (OD600 = 0.7) in fresh medium supplemented with C13-labelled urea (Sigma‒Aldrich, CAS 58069-82-2), and CaCl2 was added in an equimolar amount at 1.5 M (Table 1). This experiment was conducted in closed vials in triplicate. Thirty millilitres of CO2, corresponding to 1.24 × 10−3, was injected into each vial via a 30 mL syringe. The samples were incubated for four days at 30 °C and 100 rpm. Daily feedings of the growth medium were performed, and pH was corrected to above a value of 8. The precipitate in this test was used to determine the origins of the carbon in the formed CaCO3.
The available CO2 in the liquid phase was calculated on the basis of Henry’s law considering Henry’s law constants and the gas partial pressure24.
Precipitate analysis
The collected samples were centrifuged for 4 min at 624 × g, and the pellet was washed with acetone (Sigma Aldrich, 99% purity). After the pellet was dried overnight at 60 °C, a second washing step with 96% ethanol was performed. The samples were subsequently dried and heated at 450 °C for two hours before Fourier transform infrared (FTIR) spectroscopy (Spectrum two, Perkin Elmer) and scanning electron microscopy (SEM) analyses with energy dispersive X-ray spectroscopy (EDX) (Phenom XL, Thermo Scientific) were performed. The organic and inorganic origins of the CaCO3 precipitate was investigated by analysing the C13 -labelled fraction of carbonate via the double-capsule technique described in20.
Results
CaCO3 production in the U test
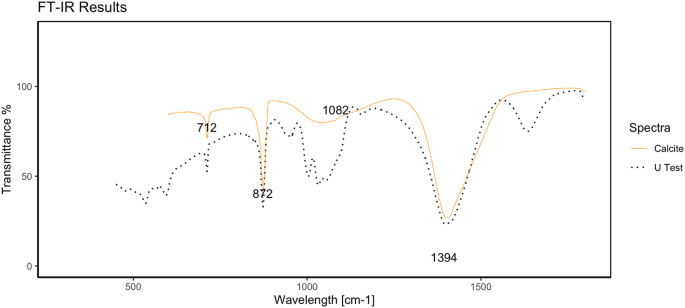
The FTIR spectra of the precipitates from the U test are reported in Fig. 2. The yellow line represents the calcite spectrum acquired from a reference database25. The wavelength range between 400 and 1500 nm contains characteristic peaks for calcite: the absorptions at 712 cm−1, 872 cm−1, 1082 cm−1, and 1394 cm−1 are influenced by the different ways in which C-O bonds form carbonate26. The black dotted line corresponds to the precipitate sample from the U test, and as expected, the characteristic peaks at 712 cm−1, 872 cm−1, and 1394 cm−1 are identified and in agreement with the reference spectrum. Additionally, in the spectrum at approximately 1082 cm−1, it is possible to distinguish a wider peak that spans several peaks. We postulate that this is due to the trace amount of other inorganic material that was still present in the pellet despite the washing procedure.
CaCO3 production in the CA test
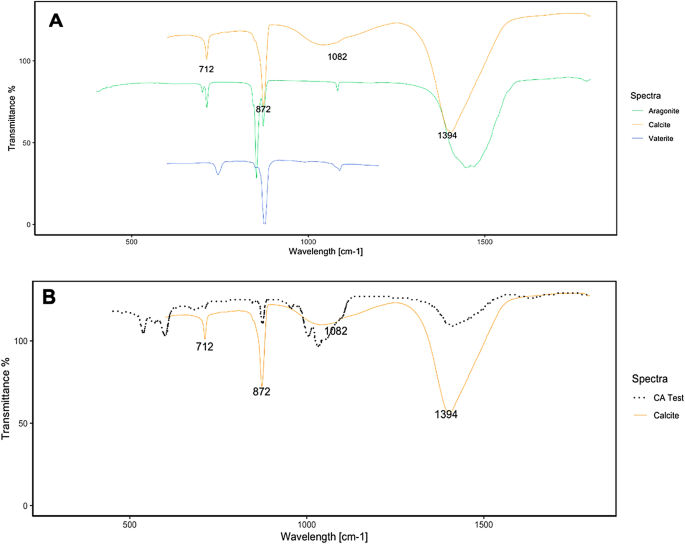
The FTIR spectra of carbonate from CA test are reported in Figure 3B. Similar to that in the previous experiment, the precipitate was identified as CaCO3 by EDX (see Supplementary Figure, S1). To elucidate the polymorphic phase of CaCO3, the spectra were subsequently analysed and compared: calcite and aragonite data were collected from the reference database (Perkin Elmer Spectrum IR), whereas the spectra of vaterite were taken from the work of27 (Figure 3A). As mentioned above, calcium carbonate has three characteristic IR absorption peaks in the range between 400 cm and 1 and 1500 cm−1 due to the interaction of the C-O bonds with infrared radiation. CaCO3, in fact, has three crystalline structures, all with the same chemical composition: calcite, aragonite, vaterite, and each crystalline structure can be associated with a corresponding spectrum (Figure 3A). A comparison of the calcite and aragonite FTIR spectra (Figure 3A) revealed that in the aragonite absorption spectra, the peak at 712 cm−1 is shifted, with an additional peak at 700 cm−1; this peak is not present in the calcite phase. In both the vaterite and calcite spectra (Figure 3A), the peak at 875 cm−1 is matched; on the other hand, the peak at 712 cm−1 present in calcite is shifted to 748 cm−1 for vaterite28. Furthermore, the third characteristic peak of calcite (at 1082 cm−1) is slightly shifted in the vaterite sample. In the CA test, the obtained spectra are consistent with the peaks corresponding to calcite (Figure 3A), which confirms that B. megaterium-induced MICP leads to the formation of calcite27.
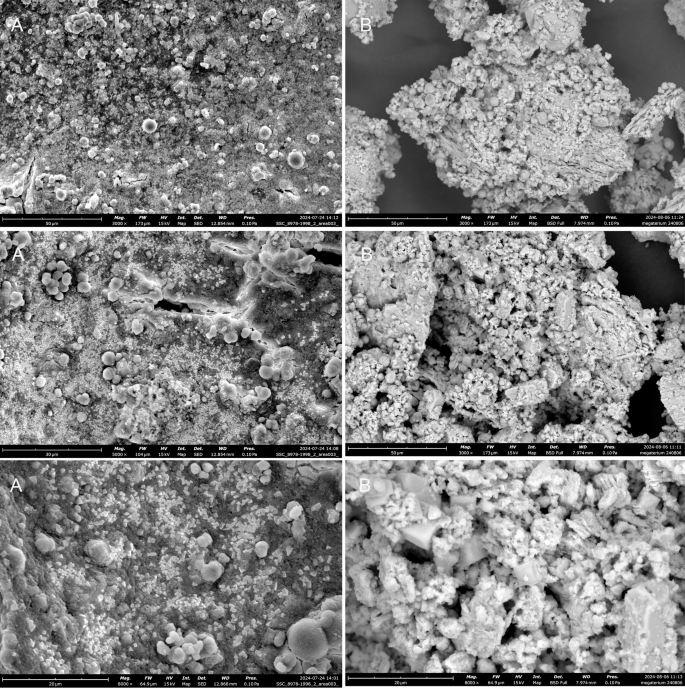
In addition to FTIR analysis, using the pellet collected from the CA Test, the textural and morphological characteristics of the crystals formed by B. megaterium were investigated using SEM. In the samples collected from the fresh medium (Figure 4A) and from the double-washed, dried pellet (Figure 4B), no dominant morphological or textural features were identified, and B. megaterium cells could not be distinguished. This could be explained by the predominant distribution of the microorganisms in the supernatant rather than in the pellet collected from the bottom of the flask after centrifugation. In the washed and dried precipitate (Figure 4B), as expected, no microbial cells were identified, but a clearer and more hierarchical matrix was observed for the residual crystals that resisted the washing and drying process. More precisely, the inorganic fraction had different morphological structures, i.e., rhombohedral, spherical, and amorphous. We postulate that the applied protocols resulted in the coexistence of various polymorphs of calcium carbonate, as observed in other MICP studies14. Therefore, it can be concluded that the final polymorph of calcium carbonate formed depends on the amount of dissolved organic carbon (DOC) present. When DOC is present in low quantities, calcite is more likely to form, whereas when DOC is present in high quantities, the formation of vaterite is favoured instead8.
The results from duplicate analyses using the double-capsule technique20 allowed for the quantification of the fraction of CaCO3 that contained the C13-labelled organic precursor (urea). Notably, 94.1% (mean value, with a standard deviation of ± 0.1%) of the total carbon was unlabelled, which implied that the vast majority of the precipitated CaCO3 was formed from the incorporation of inorganic carbon, i.e., CO2 was identified as the main carbon source and precursor of the mineral phase. This result highlights that in the presence of increased concentrations of CO2 (1.24 × 10−3), B. megaterium tends to exploit its carbonic anhydrase metabolic pathway predominantly instead of the ureolytic pathway for the synthesis of CaCO3.
Conclusions
On the basis of the results presented in this work, it can be inferred that Bacillus megaterium (DSM 32) selectively activates urease- or anhydrase-driven metabolic pathways for the precipitation of CaCO3. The pathway depends on the conditions provided and medium composition. In the presence of urea, CaCl2 and CO2 at atmospheric levels, the urease pathway is favoured. This result was a fundamental starting point for subsequent experiments in which the growth conditions were altered by incorporating increased levels of CO2 into the medium. Under these conditions, the microorganisms activated the enzyme carbonic anhydrase, and the resulting minerals were linked to CO2 at a fraction of at least 94% of the total mineral yield. This inorganic origin of the carbon that was incorporated into CaCO3 leads to the conclusion that B. megaterium preferentially activates the carbonic anhydrase pathway under the provided conditions. Another important dimension of this finding, in addition to the obvious advantages of the direct sequestration of atmospheric CO2 into CaCO3, is the avoidance of potentially hazardous metabolic byproducts produced via the urease pathway, especially ammonia, which would require additional wastewater treatment steps. The findings of this work, along with the proposed protocol for the growth of B. megaterium, can have a significant impact on the future design of microbially induced carbon dioxide sequestration (MICO2S) strategies and the incorporation of CaCO3 as a binder mineral in building applications.
Discussion
In this work, we used a known protocol27 for the growth and application of Bacillus megaterium for the precipitation of CaCO3 and benchmarked its efficiency using urea-driven MICP in open-flask experiments. The protocol was subsequently modified to allow for the incorporation of high concentrations of CO2 in a closed system, and we focused on the qualitative and quantitative analysis of the resulting precipitates. This was performed via textural observations via SEM and the use of the double-capsule technique20 to distinguish the origins of the carbon present (organic or inorganic) in the precipitate. In a closed system and under CO2 concentrations that exceeded 2300 times the concentration of CO2 in an open-air flask, i.e., atmospheric concentrations, the precipitated calcite was predominantly linked to CO2 mineralization, whereas urea labelled with C13 showed only a minor contribution (approximately 6%) to the resulting mineral as a precursor. These findings suggest a new protocol to potentially disassociate the ureolytic pathway with MICP and elucidates a new pathway for the potential valorisation of CO2 and its incorporation into mineral agents that can find multiple applications in real-world environments. The potential for bioremediation of surfaces in the built environment, including new buildings and monuments, presents promising opportunities, among others in heritage conservation. Microbial communities can self-inoculate, facilitating the consolidation and protection of stone materials29. Moreover, studies have demonstrated that the interaction between water availability and microbial communities significantly influences the degradation and conservation of stone monuments, supporting the broader application of this approach in bio-restoration practices30,31. By harnessing these processes, this methodology can contribute to sustainable conservation efforts and the mitigation of environmental damage to valuable structures. Overall, this study sets the foundation for understanding the kinetics of CO₂ mineralization and the relative contributions of different metabolic pathways, with future work potentially incorporating the use of enzymatic inhibitors or exploring the effects of varying CO₂ concentrations to further elucidate the mechanistic details of the process. As a proposed direction for future research, we recommend a detailed investigation of local pH dynamics and kinetic profiling of B. megaterium enzymatic activity under mildly acidic to neutral or alkaline conditions to better characterize its biomineralization dynamic and potential.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Phillips, A. J. et al. Engineered applications of ureolytic biomineralization: a review. Biofouling 29 (6), 715–733 (2013).
Terzis, D. & Laloui, L. A decade of progress and turning points in the understanding of bio-improved soils: A review. Geomechanics for Energy and the Environment, 19, p.100116. (2019).
Mori, D. & Uday, K. V. A review on qualitative interaction among the parameters affecting ureolytic microbial-induced calcite precipitation. Environmental Earth Sciences, 80(8), p.329. (2021).
Harran, R., Terzis, D. & Laloui, L. Mechanics, modeling, and upscaling of biocemented soils: a review of breakthroughs and challenges. International Journal of Geomechanics, 23(9), p.03123004. (2023).
Murugan, R., Suraishkumar, G. K., Mukherjee, A. & Dhami, N. K. Influence of native ureolytic microbial community on biocementation potential of Sporosarcina pasteurii. Scientific Reports, 11(1), p.20856. (2021).
Mwandira, W. et al. An electrokinetic-biocementation study for clay stabilisation using carbonic anhydrase-producing bacteria. Environ. Sci. Pollut. Res. 30 (47), 104916–104931 (2023).
Kahani, M., Kalantary, F., Soudi, M. R., Pakdel, L. & Aghaalizadeh, S. Optimization of cost effective culture medium for Sporosarcina pasteurii as biocementing agent using response surface methodology: up cycling dairy waste and seawater. J. Clean. Prod. 253, 120022. https://doi.org/10.1016/j.jclepro.2020.120022 (2020).
Dhami, N. K., Reddy, M. S. & Mukherjee, A. Bacillus megaterium mediated mineralization of calcium carbonate as biogenic surface treatment of green Building materials. World J. Microbiol. Biotechnol. 29 (12), 2397–2406. https://doi.org/10.1007/s11274-013-1408-z (2013).
Lee, J., Kim, C. G. & Mahanty, B. Mineralization of gaseous CO2 by Bacillus megaterium in close environment system225pp.1–8 (Water, Air, & Soil Pollution, 2014).
Gilmour, K. A. et al. Microbially induced calcium carbonate precipitation through CO2 sequestration via an engineered Bacillus subtilis. Microb. Cell. Fact. 23 (1). https://doi.org/10.1186/s12934-024-02437-7 (2024).
Boyke, B. et al. A short story about a big magic bug. Bioeng. Bugs 1(2), 85–91 (2010).
Biedendieck, R., Knuuti, T., Moore, S. J. & Jahn, D. The beauty in the beast—the multiple uses of Priestia megaterium in biotechnology. Appl. Microbiol. Biotechnol. 105, 5719–5737 (2021).
Wu, J. et al. Endophytic Bacillus megaterium BM18-2 Mutated for Cadmium Accumulation and Improving Plant Growth in Hybrid Pennisetum24p.e00374 (Biotechnology reports, 2019).
Carter, M. S. et al. Microbially Induced Calcium Carbonate Precipitation by Sporosarcina pasteurii: a Case Study in Optimizing Biological CaCO3 Precipitation. In Applied and Environmental Microbiology (Vol. 89, Issue 8). American Society for Microbiology. (2023). https://doi.org/10.1128/aem.01794-22
Castro-Alonso, M. J. et al. R., Microbially induced calcium carbonate precipitation (MICP) and its potential in bioconcrete: Microbiological and molecular concepts. In Frontiers in Materials (Vol. 6). Frontiers Media S.A. (2019). https://doi.org/10.3389/fmats.2019.00126
Gupta, S. K., Soni, S. K. & Gupta, R. K. Effects of pH and temperature on calcium carbonate precipitation by urease activity of Sporosarcina pasteurii. Water Environ. Res. 90 (12), 2011–2020. https://doi.org/10.1111/wej.12467 (2018).
Rodriguez-Blanco, J. D., Shaw, S., Bots, P., Roncal-Herrero, T. & Benning, L. G. Transformation of amorphous calcium carbonate into crystalline polymorphs: influence of mg²⁺ and pH. Eur. J. Mineral. 27 (6), 717–726. https://doi.org/10.2138/em-2015-5294 (2015).
Tobler, D. J., Rodriguez-Blanco, J. D., Dideriksen, K., Sand, K. K. & Benning, L. G. The role of pH and Mg on the stability and crystallization of amorphous calcium carbonate. J. Alloys Compd. 654, 485–490. https://doi.org/10.1016/j.jallcom.2015.09.108 (2016).
Sun, X. & Miao, L. Application of bio-remediation with bacillus megaterium for crack repair at low temperature. J. Adv. Concr. Technol. 18 (5), 307–319. https://doi.org/10.3151/jact.18.307 (2020).
Tumiati, S. et al. Subducted organic matter buffered by marine carbonate rules the carbon isotopic signature of Arc emissions. Nat. Commun. 13 (1). https://doi.org/10.1038/s41467-022-30421-5 (2022).
Shi, W. et al. Study of concrete Crack repair using Bacillus megaterium. Advances in Materials Science and Engineering, 2022(1), p.6188680. (2022).
Manna, S., Dutta, S., Kumar, M., Panwar, A. & Roy, D. Urease-driven CaCO3 production by bacillus megaterium RB-05 for application in sand stabilization. Biologia 79 (9), 2907–2915 (2024).
Gallert, C., Bauer, S. & Winter, J. Effect of ammonia on the anaerobic degradation of protein by mesophilic and thermophilic Biowaste populations. Appl. Microbiol. Biotechnol. 50 (4), 495–501. https://doi.org/10.1007/s002530051326 (1998).
Sander, R. Compilation of henry’s law constants (version 4.0) for water as solvent. Atmos. Chem. Phys. 15 (8), 4399–4981 (2015).
Veerasingam, S. & Venkatachalapathy, R. Estimation of Carbonate Concentration and Characterization of Marine Sediments by Fourier Transform Infrared Spectroscopy66pp.136–140 (Infrared Physics & Technology, 2014).
Matei, C., Berger, D., Dumbrava, A., Radu, M. D. & Gheorghe, E. Calcium carbonate as silver carrier in composite materials obtained in green seaweed extract with topical applications. J. Solgel Sci. Technol. 93, 315–323 (2020).
Kaur, G., Dhami, N. K., Goyal, S., Mukherjee, A. & Reddy, M. S. Utilization of carbon dioxide as an alternative to Urea in biocementation. Constr. Build. Mater. 123, 527–533. https://doi.org/10.1016/J.CONBUILDMAT.2016.07.036 (2016).
Wu, Y. et al. Flower-like vaterite produced by nanobubble-containing ethanol and water mixed solution for Cd (II) removal. Advanced Powder Technology, 35(1), p.104279. (2024).
Jroundi, F. et al. Protection and consolidation of stone heritage by self-inoculation with indigenous carbonatogenic bacterial communities. Nature communications, 8(1), p.279. (2017).
Elert, K. et al. Degradation of ancient Maya carved tuff stone at Copan and its bacterial bioconservation. npj Materials Degradation, 5(1), p.44. (2021).
Wang, L. et al. Water determines geomicrobiological impact on stone heritage. Nature Geosci. 18, 108–111 (2025).
Acknowledgements
The authors express their sincere thanks to the Swiss Innovation Agency (Innosuisse) for their support through the innovation project Ref: 02.649 IP-ENGMiBOC.
Ethics declarations
Competing interests
The authors declare that there are no competing interests related to this publication. The research, analysis, and findings presented are independent of any financial, professional, or personal relationships that could influence the content or interpretation of the results.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cappa, M., Perego, C., Terzis, D. et al. Bacillus megaterium favours CO₂ mineralization into CaCO₃ over the ureolytic pathway. Sci Rep 15, 21861 (2025). https://doi.org/10.1038/s41598-025-07323-9
Received: 26 January 2025
Accepted: 13 June 2025
Published: 01 July 2025
DOI: https://doi.org/10.1038/s41598-025-07323-9
.png)