- Article
- Open access
- Published: 16 April 2025
Scientific Reports volume 15, Article number: 13051 (2025) Cite this article
-
3219 Accesses
-
98 Altmetric
Abstract
Gambling behaviour is a persistent and growing societal problem. An unexplored factor that may encourage gambling behaviour is the impact of circadian photoreception on cognitive processes underlying the behaviour. We investigated the influence of circadian photoreception on loss aversion in gambling by altering the blue content of light while maintaining the same visual brightness. Fifteen participants (age 18–27 years, M = 20.40, SD = 2.03) completed an economic decision-making task under blue-enriched and blue-depleted light, of equivalent visual brightness, on separate occasions in a randomised order. The task required participants to choose between taking a risky gamble of a positive and negative outcome, or a less risky guaranteed outcome. Hierarchical Bayesian Modelling was conducted to derive individual parameter estimates for loss aversion, and trial-by-trial performance was analysed using linear mixed models. The findings demonstrated that individuals were significantly less loss averse under blue-enriched light compared to blue-depleted light (β = − .43, 95% CI [− .82, − .04], p = .03). This study shows that exposure to light that preferentially targets circadian photoreception reduces loss aversion, which may encourage gambling behaviour.
Similar content being viewed by others
Introduction
Approximately 1.6 billion people worldwide engage in gambling activities1. The widespread availability of smartphones and computers has led to a surge in online gambling (e.g., sports betting), with the online gambling market projected to reach over US$136 billion by 20292. The manipulation of gambling product designs can significantly influence gambling behaviour. For example, sound effects commonly associated with gambling, like the ringing bell on a slot machine following a win, have been shown to reinforce gambling behaviour3. Additionally, the use of light and colour may further exacerbate gambling behaviour4,5.
Light has profound effects on cognition, enhancing alertness and attention through direct and indirect input to multiple brain regions6,7. Intrinsically photosensitive retinal ganglion cells (ipRGCs), a group of specialised retinal ganglion cells, contain the photopigment melanopsin and respond preferentially to short wavelength ‘blue’ light (~ 480 nm)8. Blue-enriched light typically exhibits the most considerable non-visual effects and is most effective for improving alertness, attention9,10 and subjective wellbeing6. ipRGCs convey non-visual, largely non-conscious effects of light, projecting to brain regions involved in inhibitory control, risky decision-making, and emotion-regulation, including the inferior frontal gyrus,dorsal prefrontal cortex11, and amygdala12. Light exposure can lead to suppression of activity in the amygdala12, which plays an important role in motivation and sensitivity to reward. The ability of light to suppress amygdala activity may reduce fear-related effects commonly associated with gambling. Furthermore, decreased activity of the habenula, a brain region involved in reward regulation13,14, is associated with increased expectation of reward13, and has been shown to be impacted by light exposure in humans15. The ability of ipRGCs to suppress brain regions involved in decision-making and reward may enhance the encoding of rewarding stimuli while diminishing responses to loss. Consequently, light that preferentially activates ipRGCs could alter how likely someone is to engage in risky decision-making.
When individuals need to make a decision that involves risk and uncertainty (as per “Prospect Theory”), they are likely to value losses and gains disproportionately16,17. Prospect Theory suggests that we evaluate outcomes based on their relative utility, rather than absolute utility16,17. Therefore, individuals are more likely to feel worse about losing $100 than feel good about gaining $100. Loss aversion, a key component of Prospect Theory, refers to the preference for certainty over uncertainty16,17, whereby the psychological pain of losing is stronger than the pleasure of gaining16,17. Loss aversion is modelled by a value function with an asymmetric ‘S’ curve16. In this model, gains and losses are defined relative to a reference point, often called the status quo, and the slope of the value function for losses is steeper than that for gains17. In gambling, reduced loss aversion is often associated with riskier gambling patterns18,19.
Using computational modelling, choice data in gambling tasks can be explained in terms of underlying parameters20. The loss aversion coefficient, lambda (λ), represents the weighting of the psychological value of losses compared to gains16,21. When λ is less than 1, gains are considered more valuable than losses, indicating a propensity for seeking gains22. Conversely, when λ exceeds 1, losses are perceived as more significant than gains, indicating heightened loss aversion22. By utilising such computational modelling techniques, we can evaluate an individual’s responsiveness to loss and gain insights into how light exposure influences loss aversion.
Given that decision-making is influenced by underlying cognitive processes that are directly impacted by circadian photoreception, we anticipated that activation of circadian photoreceptors would alter an individual’s sensitivity to loss. In this study, we tested the hypothesis that when exposed to blue-enriched light, individuals would exhibit lower levels of loss aversion, as indicated by higher λ values, compared to when exposed to blue-depleted light of the same visual brightness.
Method
Participants
A total of 15 young, healthy adults (five men) aged 18–27 years (M = 20.40, SD = 2.03) provided informed consent and completed this within-subjects study. All participants completed both light conditions, with the order of blue enriched/depleted light randomly assigned. Participants were free from major medical conditions, were not taking regular prescription medications, and had no personal history of psychiatric conditions. Participants were largely classed as intermediate types on the Morningness-Eveningness Questionnaire23 (60% intermediate, M = 44.13, SD = 7.57). Mean self-reported bedtime and waketime were 24.76 and 8.50 in decimal clock time (SD = 1.62 and 1.38, respectively). The Monash University Human Research Ethics Committee (MUHREC) approved the study (Project #32054), and the study was conducted in accordance with the relevant guidelines and regulations.
Materials
Melagen lighting device
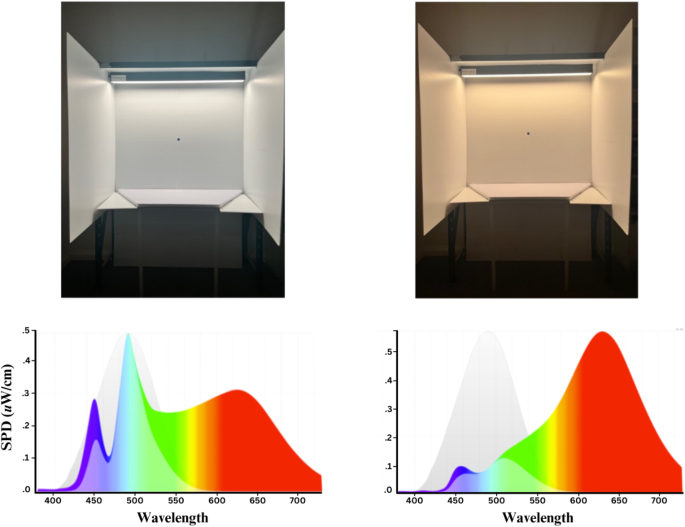
Light exposure was controlled using Melagen lighting (Versalux Lighting, Mitcham, VIC, Australia). The device is an LED light source (CR189), where participants are exposed to one of two lighting conditions: blue-depleted (peak wavelength of ~ 630 nm and colour temperature of ~ 2700 K) and blue-enriched (peak wavelength of ~ 485 nm and colour temperature of ~ 6500 K). The two lighting conditions were delivered at different melanopic equivalent daylight illuminance (~ 191.23 lx for the blue-enriched condition and ~ 77.57 lx for the blue-depleted condition), but the same photopic illuminance (visual brightness) of ~ 200 lx. The photopic illuminance of 200 lx for both conditions was chosen as it aligns with typical office settings24, and as melatonin suppression is an indicator of melanopsin activation, photopic illuminance of 200 lx indicates that ipRGCs (which contain melanopsin) are activated. See Fig. 1 for a visual representation of the two lighting conditions’ spectral qualities and visual differences.
The visual appearances and spectral distributions of the blue-enriched light condition (left) and blue-depleted light condition (right). Spectral power distributions (SPDs) were generated by the software f.luxometer LLC (Los Angeles, California, USA) and show the light’s spectrum (coloured area), the melanopic action spectrum (grey curve with peak at ~ 480 nm), and the relative melanopic activation due to the light source (lightened area under coloured spectrum).
Economic decision-making task
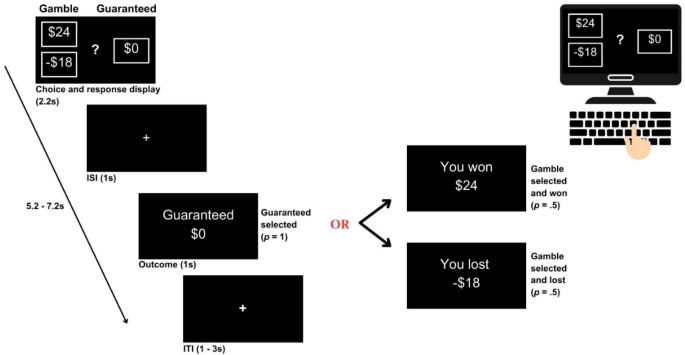
Participants completed the Economic Decision-Making task to assess loss aversion, an adaptation of the Loss Aversion Task22. Participants were asked to take a risky gamble between a positive and a negative outcome or take a less risky guaranteed outcome (see Fig. 2 for a task schematic). After each decision was made, participants were informed of their result (e.g., “you won $10”, “you lost $2.50”, or “trial missed” if they failed to respond within 2 s of the signal). Participants were given a practice block of 7 trials, and once they confirmed their understanding of the task, they were presented with a further 140 trials. One hundred and twenty trials contained a mixture of positive and negative outcomes (e.g., win or lose), and 20 trials were positive gain-only gambles. Before completing the task, participants were informed of a $20 bonus, which was contingent upon their performance.
A schematic of the Economic Decision-Making Task. Participants were asked to make monetary choices between gambles (winning and losing with equal probability = .5) and a guaranteed alternative (probability = 1), with the outcome following each choice. Participants were presented with the gamble and guaranteed amount and were required to accept the gamble or reject it for the guaranteed alternative (2.2 s). After an interstimulus interval (ISI) of 1 s, the outcome screen was presented consisting of a win or lose screen with equal probability if the gamble had been accepted, otherwise the guaranteed alternative if the gamble had been rejected (1 s). An intertrial interval (ITI) of 1–3 s separated each trial from the next. The schematic was generated by Canva.com.
Procedure
Before attending the laboratory session, consented participants were asked to obtain a normal night’s sleep and abstain from alcohol and caffeine for 24 h before each visit. Laboratory visits took place between four and seven hours after the participants’ usual waketime, to avoid confounding by potential time of day or sleep inertia effects. The two sessions were completed at least two weeks apart (on average 14.5 days after the participant’s first visit).
Upon arrival, participants were seated under the light device, with distance and height standardised by eye-level of 123 cm from the floor and 63.3 cm from a fixation point. Participants completed an initial ‘dark’ period (< 3 lx) for five minutes, and then completed the rest of the session under either the blue-enriched or blue-depleted light condition. Lighting condition order was randomised for the two sessions (blue-enriched-blue-depleted = 9 and blue-depleted-blue-enriched = 6). After ~ 34 min of light exposure, participants were asked to complete the economic decision-making task as quickly and accurately as possible (~ 10 min in duration). Participants were reimbursed up to $100 for their participation ($50 for study completion and an additional $50 dependent on task performance). Participants were required to reach a certain threshold to receive the additional compensation. The threshold was designed to enhance participation motivation; however, it was set at a level whereby all participants received the full payment.
Behavioural analysis and results
All statistical analyses were performed using R Statistical Software (v4.3.1)25. Hierarchical Bayesian Modelling was used to investigate differences between lambda (λ; loss aversion parameter) and the light conditions. Hierarchical Bayesian Modelling is based on the Prospect Theory framework, whereby loss aversion is formalised in accordance with the general formula16:
$$u\left( {x^{ + } } \right) = x^{\rho }$$
$$u\left( x \right) = - \lambda \times \left( { - x} \right)^{\rho }$$
In this formula, u is the logit sensitivity, x is the outcome, λ represents the loss aversion coefficient and ρ is the curvature of the utility function.
Hierarchical Bayesian Modelling estimates the lambda parameter for individuals and pools information across individuals for group-level (condition) data. This results in “shrinkage” effects, where individual estimates inform the group estimates and sequentially inform the estimates of individual parameters21.
The hBayesDM package in R was used to estimate lambda coefficients (λ), the loss aversion parameter. Parameter estimates were obtained using a hierarchical Bayesian method that uses Markov-chain Monte Carlo (MCMC) sampling to generate full posterior distributions of model parameters. Models separated by sex were fit, and individual parameter values (λ) were computed using the ra_noRA function. The ra_noRA function used 10,000 iterations to produce λ values for individuals in light conditions (blue-enriched and blue-depleted). Convergence of the Markov chains was determined by visual inspection of the chains for all parameters. Convergence was also quantitatively diagnosed to ensure that samples were adequately mixed and converged, indicated by trace plots and Rhat values < 1.1.
Summary behavioural data
Under blue-enriched light, males exhibited an average propensity to choose the gamble option in 60.27% of trials, compared to 39.76% for females. Under blue-depleted light, males demonstrated a slightly lower propensity (55.15%), as did females (36.05%). Therefore, males were more likely to choose the riskier option across both light conditions, but the differences between conditions were not statistically significant (p > .05; see Table 1). In addition, response times for guaranteed and gamble options did not significantly differ between blue-enriched and blue-depleted light (see Table 1 for a summary).
Loss aversion (λ) parameter estimation
Under blue-enriched light, the computed mean (with 95% HDI) for men was λ = .79, [.56, 1.06]. As λ < 1, under blue-enriched light, men tended to be more gain-seeking than loss averse. In comparison, the computed mean (with 95% HDI) for women was λ = 1.39, [1.08, 1.77]. As λ > 1, women under blue-enriched light were more loss averse than gain-seeking.
Under blue-depleted light, the computed mean (with 95% HDI) for men was λ = .82, [.63, 1.05], indicating that men were gain-seeking under blue-depleted light (λ < 1). In comparison, the computed mean (with 95% HDI) for women was λ = 2.01 (1.45, 2.66), indicating that women were highly loss aversive under blue-depleted light (λ ≫ 1). There was no meaningful difference between the groups.
Effects of light condition and sex on loss aversion parameters
Linear mixed-effects models were fit to investigate the influence of light conditions (blue-enriched and blue-depleted light) on loss aversion (λ) parameters, estimated and analysed on a trial-by-trial basis and within-subjects (estimated using restricted maximum likelihood (REML) and nloptwrap optimizer). Random intercepts for each individual were included to control for individual differences across conditions (formula: ~ 1|participant). We included ‘condition’ (blue-enriched or blue-depleted light), ‘sex’ and the interaction between ‘condition’ and ‘sex’ (condition*sex) as predictor variables in a possible set of models (see Table 2 for model comparison). The best overall model was assessed using the Akaike Information Criterion corrected for small sample sizes (AICc)26. A Wald t-distribution approximation method was used to calculate 95% confidence intervals and p-values. Assumptions of linearity, homoscedasticity and normal distribution of the y-intercept were met. Although the normal distribution of the outcome variable was not met, it did fit the expected distribution of lambda, and thus no transformation was conducted.
The best overall model included the intercept, condition, and sex. The model explained a majority of the variance, as indicated by the conditional R2 value of .66. The proportion of variance explained by the fixed effects alone was 31%, as indicated by marginal R2. The ICC indicated that 50% of the total variance in λ (loss aversion) was attributable to between-person differences (τ00 = 0.27). See Table 3 for model parameter estimates.
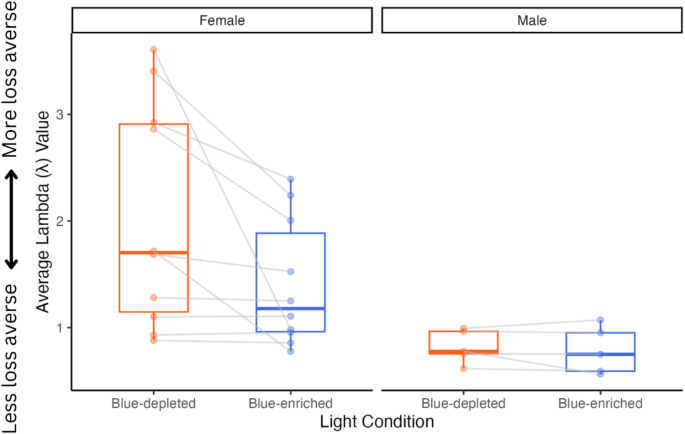
For the best-fit model, the intercept indicates that women in the blue-depleted condition had an average loss aversion parameter of λ = 1.94. Light condition significantly affected the loss aversion parameter, whereby blue-enriched light condition was associated with a − .43 average decrease in loss aversion (see Fig. 3). Conceptually, the loss aversion parameter can be viewed as an equivalent monetary amount in dollars. Therefore, under blue-depleted light, women participants required a potential win of $204 (λ = 2.04) to risk losing $100. When exposed to blue-enriched light, λ decreased by 0.43 (λ = 1.51). Therefore, women required a lower potential win of $151 to risk losing $100 under these conditions. Additionally, sex significantly affected the loss aversion parameter, with a.92 decrease in the loss aversion parameter (λ = 1.02) in men.
Discussion
We investigated the potential influence of circadian photoreception on loss aversion during a gambling task, by manipulating the melanopic brightness of light while controlling for visual brightness. Our results revealed a decrease in loss aversion under blue-enriched light, which has a greater impact on circadian photoreceptors. We also found significant differences in loss aversion between sexes across the two light conditions, with women displaying greater loss aversion compared with men.
Individuals tend to have an unequal balance of evaluations between losses and gains, with losses typically outweighing gains for subsequent behaviour16. Under blue-enriched light, our findings reveal that individuals are less loss averse, suggesting that when under such light, individuals are relatively more comfortable with taking risks and demonstrate a reduced sensitivity to losses compared to gains of equal magnitude. In a gambling scenario, individuals under blue-depleted light might perceive the subjective impact of a $100 loss as significantly larger than the potential $100 gain. However, under blue-enriched light, the subjective impact of a $100 loss may not be as large, leading individuals to have less negative reaction to losses. Manipulations that alter this balance are important for subsequent behaviour as this could affect whether someone decides to continue engaging in gambling activities or discontinuing.
Our findings demonstrate that individuals exhibit reduced loss aversion under blue-enriched light, while controlling for visual brightness (~ 200 lx for both conditions). As both the spectral quality and intensity of light can impact cognition and several brain regions9,10,12,27, by controlling the visual brightness, we were able to isolate the specific influence of blue content on circadian photoreceptors, which are known to affect brain regions associated with decision-making. It is possible that reduced loss aversion under blue-enriched light may be due to the effect of light, via ipRGCs (which preferentially respond to blue light8), on specific brain regions. In rodents, ipRGCs have been found to innervate the amygdala28, a structure that plays a pivotal role in reward processing29 and is part of an impulsive system that triggers emotional responses to immediate outcomes30. The amygdala is believed to play an important role in evaluating the subjective appeals and disadvantages of potential gains and losses during mixed gambles31. Previous work has found an association between reduced amygdala response to loss and emotional regulation strategies, including reappraisal (i.e., reframing how individuals think about outcomes)22,32, with damage to the amygdala reducing loss aversion33. As bright light suppresses amygdala activity12, exposure to blue-enriched light may reduce negative emotions and the ability to evaluate subjective appeals, resulting in individuals being less averse to potential losses.
In addition to the amygdala, the habenula plays a role in the decision-making process, including reward regulation13,34. Animal studies suggest that the habenula deters behaviours associated with negative outcomes (e.g., punishment), while reinforcing those linked to positive outcomes, influencing motivation and decision-making35. Specifically, the lateral habenula is implicated in reward prediction. Neurons in the lateral habenula encode negative reward prediction error and are activated by unexpected, non-rewarding and unpleasant events while being suppressed by unexpected rewarding events14. Heightened activation of the lateral habenula may inhibit individuals from accepting risky gambles, guiding them towards safer outcomes and potentially increasing their loss aversion. In humans, light has been found to directly impact habenula activation in fMRI studies15. Through direct input from retinal ganglion cells and other brain structures involved in non-visual photoreception, the impact of light on habenula activation may lead to an elevated expectation of reward, reducing the psychological “pain” typically associated with losses and making individuals less averse to loss35.The brain’s reward system likely contributes to the observed reduction in loss averse behaviour under blue-enriched light. Dopamine, a key neurotransmitter involved in daily functioning, plays a central role in the reward system by processing reward information36,37. Dopamine neurons exhibit responses relative to reward prediction, with unexpected rewards triggering increased activation and expected rewards leading to maintained or decreased activity37,38. Dopamine neurons project to brain regions such as the insula and striatum, where heightened activity in response to gains causes a reduced sensitivity to loss31. These regions are implicated in circadian photoreception, with bright light exposure enhancing their activation7,39,40. This suggests that light can boost activity in reward-related brain regions, potentially amplifying the influence of the reward system. Consequently, individuals may value potential rewards more and find riskier decisions more appealing under blue-enriched light.
In addition to the observed effects of light on decision-making processes, we observed substantial interindividual differences in loss aversion. Our findings revealed lower loss aversion among men, consistent with existing literature highlighting sex differences in loss aversion41,42,43,44. Women often experience more negative emotions (e.g., fear), leading to a lower willingness to undertake financial risk (e.g., invest less money or gamble less)45,46. Conversely, men tend to experience more positive emotions associated with gambling (e.g., optimism and confidence), which may predispose them to underestimate negative outcomes45,46,47. Furthermore, neurobiological differences may play a role, as men and women have shown disparities in brain regions associated with decision-making. For example, men often exhibit activation in regions such as the lateral orbitofrontal cortex (OFC) and DLPFC during risky decision-making, whereas women may show activation primarily in a smaller region of the left medial OFC41,48. Therefore, the observed sex disparities in loss aversion may arise from a complex interplay between emotions and neurobiological differences in brain activation patterns.
There are substantial interindividual differences in light sensitivity mediated by circadian photoreception. Previous work has found a greater than 50-fold difference in sensitivity to evening light, with some individuals exhibiting > 50% melatonin suppression (a marker of light sensitivity) in response to dim light (~ 10 lx), while less sensitive individuals required ~ 400 lx (equivalent to bright office lights) to achieve the same melatonin suppression49. Several factors are associated with individual differences in light sensitivity, with younger individuals exhibiting higher sensitivity to light compared with older populations50,51,52. Additionally, increased sensitivity of the circadian system to light has been found among individuals with bipolar disorder, with increased sensitivity to light suggested to be a trait marker of bipolar disorder48,49. Younger adults are more likely to engage in risky gambling behaviour53,54,55,56, and the risk of problem gambling is four times higher in patients with bipolar disorder than the general population57. If gambling behaviour and loss aversion are influenced by circadian photoreception, it is likely that individuals with higher light sensitivity would experience less of an impact to loss and therefore gamble more than individuals who are less sensitive to light.
It should be noted that this study had a relatively small sample size for computational modelling58,59. However, our model diagnostics indicated that the MCMC samples converged with values less than 1.1, highlighting reliability in the model estimates19. Furthermore, the within-subjects design of our study substantially boosted statistical power. However, given the small sample size and sex imbalance, additional studies of light condition and loss aversion are needed as it is possible that this limitation may have masked potential interactions of sex by light condition on loss aversion. Furthermore, with respect to interpretation of the findings, while the visual brightness of both conditions was equivalent, we were unable to exclude the role of differences in visual experience between the light conditions.
The ability to control our light environment is a relatively recent development. In our natural history, light exposure was largely determined by the rise and fall of the sun. Humans now spend ~ 90% indoors under artificial light60, and with the growing dominance of energy-efficient LED lights, this light tends to be more blue-enriched, leading to increased activation of circadian photoreception. Virtually all machines used for gambling, including slot machines, now employ LED/LCD displays which are known for their high light intensity and blue-enriched light content. Furthermore, with the prevalence of online gambling increasing, individuals are turning to devices that are likely to emit blue-enriched light (e.g., smartphones and tablets). Exposure to blue-enriched displays possibly contributes to increased gambling behaviour, by reducing an individual’s loss aversion, thereby making them more likely to select uncertain financial outcomes over guaranteed, safer choices. Targeting the reduction of “blue” light content in gambling scenarios may be a promising target for reducing gambling behaviour by promoting greater loss sensitivity.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
Calado, F. & Griffiths, M. D. Problem gambling worldwide: An update and systematic review of empirical research (2000–2015). J. Behav. Addict. 5, 592–613 (2016).
Statista. Online Gambling—Worldwide (2023).
Dixon, M. J. et al. The impact of sound in modern multiline video slot machine play. J. Gambl. Stud. 30, 913–929 (2014).
Finlay, K., Marmurek, H. H., Kanetkar, V. & Londerville, J. Assessing the Contribution of Gambling Venue Design Elements to Problem Gambling Behaviour (University of Guelph, 2007).
Grant, J. E. & Chamberlain, S. R. Impulsive action and impulsive choice across substance and behavioral addictions: Cause or consequence?. Addict. Behav. 39, 1632–1639 (2014).
Chellappa, S. L. et al. Non-visual effects of light on melatonin, alertness and cognitive performance: Can blue-enriched light keep us alert?. PLoS ONE 6, e16429 (2011).
Vandewalle, G., Maquet, P. & Dijk, D.-J. Light as a modulator of cognitive brain function. Trends Cogn. Sci. 13, 429–438 (2009).
Berson, D. M., Dunn, F. A. & Takao, M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 295, 1070–1073 (2002).
Hung, S.-M. et al. Cerebral neural correlates of differential melanopic photic stimulation in humans. Neuroimage 146, 763–769 (2017).
Vandewalle, G. et al. Brain responses to violet, blue, and green monochromatic light exposures in humans: Prominent role of blue light and the brainstem. PLoS ONE 2, 1–10 (2007).
Aron, A. R., Robbins, T. W. & Poldrack, R. A. Inhibition and the right inferior frontal cortex. Trends Cogn. Sci. 8, 170–177 (2004).
McGlashan, E. M., Poudel, G. R., Jamadar, S. D., Phillips, A. J. & Cain, S. W. Afraid of the dark: Light acutely suppresses activity in the human amygdala. PLoS ONE 16, e0252350 (2021).
Matsumoto, M. & Hikosaka, O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature 447, 1111–1115 (2007).
Proulx, C. D., Hikosaka, O. & Malinow, R. Reward processing by the lateral habenula in normal and depressive behaviors. Nat. Neurosci. 17, 1146–1152 (2014).
Kaiser, C. et al. The human habenula is responsive to changes in luminance and circadian rhythm. NeuroImage Orlando Fla 189, 581–588 (2019).
Kahneman, D. & Tversky, A. Prospect theory: An analysis of decision under risk. Econometrics 47, 263–291 (1979).
Tversky, A. & Kahneman, D. Advances in prospect theory: Cumulative representation of uncertainty. J. Risk Uncertain. 5, 297–323 (1992).
Genauck, A. et al. Reduced loss aversion in pathological gambling and alcohol dependence is associated with differential alterations in amygdala and prefrontal functioning. Sci. Rep. 7, 16306 (2017).
Zhang, K. & Clark, L. Loss-chasing in gambling behaviour: Neurocognitive and behavioural economic perspectives. Curr. Opin. Behav. Sci. 31, 1–7 (2020).
Huys, Q. J., Maia, T. V. & Frank, M. J. Computational psychiatry as a bridge from neuroscience to clinical applications. Nat. Neurosci. 19, 404–413 (2016).
Ahn, W.-Y., Haines, N. & Zhang, L. Revealing neurocomputational mechanisms of reinforcement learning and decision-making with the hBayesDM package. Comput. Psychiatry Camb. Mass 1, 24 (2017).
Sokol-Hessner, P., Camerer, C. F. & Phelps, E. A. Emotion regulation reduces loss aversion and decreases amygdala responses to losses. Soc. Cogn. Affect. Neurosci. 8, 341–350 (2013).
Horne, J. A. & Ostberg, O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol. 4, 97–110 (1976).
Prayag, A. S., Najjar, R. P. & Gronfier, C. Melatonin suppression is exquisitely sensitive to light and primarily driven by melanopsin in humans. J. Pineal Res. 66, e12562 (2019).
R Core Team. R: A Language and Environment for Statistical Computing (2021).
Burnham, K. P. & Anderson, D. R. A practical information-theoretic approach. In Model Selection and Multimodel Inference (Springer, 2002).
Kaiser, C. et al. The human habenula is responsive to changes in luminance and circadian rhythm. Neuroimage 189, 581–588 (2019).
Hattar, S. et al. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J. Comp. Neurol. 497, 326–349 (2006).
Phelps, E. A. & LeDoux, J. E. Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron 48, 175–187 (2005).
Bechara, A. Decision making, impulse control and loss of willpower to resist drugs: A neurocognitive perspective. Nat. Neurosci. 8, 1458–1463 (2005).
Dreher, J.-C. Sensitivity of the brain to loss aversion during risky gambles. Trends Cogn. Sci. 11, 270–272 (2007).
Sokol-Hessner, P. et al. Thinking like a trader selectively reduces individuals’ loss aversion. Proc. Natl. Acad. Sci. 106, 5035–5040 (2009).
De Martino, B., Camerer, C. F. & Adolphs, R. Amygdala damage eliminates monetary loss aversion. Proc. Natl. Acad. Sci. 107, 3788–3792 (2010).
Zack, M., George, R. S. & Clark, L. Dopaminergic signaling of uncertainty and the aetiology of gambling addiction. Prog. Neuropsychopharmacol. Biol. Psychiatry 99, 109853 (2020).
Boulos, L.-J., Darcq, E. & Kieffer, B. L. Translating the habenula—from rodents to humans. Biol. Psychiatry 81, 296–305 (2017).
Lewis, R. G., Florio, E., Punzo, D. & Borrelli, E. The brain’s reward system in health and disease. In Circadian Clock in Brain Health and Disease (eds. Engmann, O. & Brancaccio, M.) vol. 1344 57–69 (Springer International Publishing, 2021).
Schultz, W. Predictive reward signal of dopamine neurons. J. Neurophysiol. 80, 1–27 (1998).
Satoh, T., Nakai, S., Sato, T. & Kimura, M. Correlated coding of motivation and outcome of decision by dopamine neurons. J. Neurosci. 23, 9913–9923 (2003).
Alkozei, A. et al. Exposure to blue light increases subsequent functional activation of the prefrontal cortex during performance of a working memory task. Sleep 39, 1671–1680 (2016).
Macoveanu, J. et al. Bright-light intervention induces a dose-dependent increase in striatal response to risk in healthy volunteers. Neuroimage 139, 37–43 (2016).
Bolla, K. I., Eldreth, D. A., Matochik, J. A. & Cadet, J. L. Sex-related differences in a gambling task and its neurological correlates. Cereb. Cortex 14, 1226–1232 (2004).
Rau, H. A. The disposition effect and loss aversion: Do gender differences matter?. Econ. Lett. 123, 33–36 (2014).
van den Bos, R., Homberg, J. & de Visser, L. A critical review of sex differences in decision-making tasks: Focus on the Iowa gambling task. Behav. Brain Res. 238, 95–108 (2013).
Villanueva-Moya, L. & Expósito, F. Gender differences in decision-making: The effects of gender stereotype threat moderated by sensitivity to punishment and fear of negative evaluation. J. Behav. Decis. Mak. 34, 706–717 (2021).
Dawson, C. Gender differences in optimism, loss aversion and attitudes towards risk. Br. J. Psychol. 114, 928–944 (2023).
Fujita, F., Diener, E. & Sandvik, E. Gender differences in negative affect and well-being: the case for emotional intensity. J. Pers. Soc. Psychol. 61, 427 (1991).
Powell, M. & Ansic, D. Gender differences in risk behaviour in financial decision-making: An experimental analysis. J. Econ. Psychol. 18, 605–628 (1997).
Lawrence, N. S., Jollant, F., O’Daly, O., Zelaya, F. & Phillips, M. L. Distinct roles of prefrontal cortical subregions in the Iowa gambling task. Cereb. Cortex 19, 1134–1143 (2009).
Phillips, A. J. K. et al. High sensitivity and interindividual variability in the response of the human circadian system to evening light. Proc. Natl. Acad. Sci. 116, 12019–12024 (2019).
Chellappa, S. L., Bromundt, V., Frey, S. & Cajochen, C. Age-related neuroendocrine and alerting responses to light. GeroScience 43, 1767–1781 (2021).
Duffy, J. F., Zeitzer, J. M. & Czeisler, C. A. Decreased sensitivity to phase-delaying effects of moderate intensity light in older subjects. Neurobiol. Aging 28, 799–807 (2007).
Herljevic, M., Middleton, B., Thapan, K. & Skene, D. J. Light-induced melatonin suppression: Age-related reduction in response to short wavelength light. Exp. Gerontol. 40, 237–242 (2005).
Buth, S., Wurst, F. M., Thon, N., Lahusen, H. & Kalke, J. Comparative analysis of potential risk factors for at-risk gambling, problem gambling and gambling disorder among current gamblers—results of the Austrian representative survey 2015. Front. Psychol. 8, 2188 (2017).
Çakıcı, M., Çakıcı, E., Babayiğit, A. & Karaaziz, M. Gambling behaviour: Prevalence, risk factors and relation with acculturation in 2007–2018 North Cyprus adult household surveys. J. Gambl. Stud. 37, 1099–1111 (2021).
Ciarrocchi, J. W. Counseling Problem Gamblers: A Self-Regulation Manual for Individual and Family Therapy (Academic Press, 2002).
Çakıcı, M., Çakıcı, E. & Karaaziz, M. Lifetime of prevalence and risk factors of problem and pathologic gambling in North Cyprus. J. Gambl. Stud. 32, 11–23 (2016).
Jones, L. et al. Gambling problems in bipolar disorder in the UK: Prevalence and distribution. Br. J. Psychiatry 207, 328–333 (2015).
Fareri, D. S., Stasiak, J. E. & Sokol-Hessner, P. Choosing for others changes dissociable computational mechanisms underpinning risky decision-making. Sci. Rep. 12, 14361 (2022).
Barkley-Levenson, E. E., Van Leijenhorst, L. & Galván, A. Behavioral and neural correlates of loss aversion and risk avoidance in adolescents and adults. Dev. Cogn. Neurosci. 3, 72–83 (2013).
Klepeis, N. E. et al. The national human activity pattern survey (NHAPS): A resource for assessing exposure to environmental pollutants. J. Expo. Sci. Environ. Epidemiol. 11, 231–252 (2001).
Acknowledgements
The work conducted was completed with the help of our participants and research staff. This research is funded by the Australian Government through the Australian Research Council (DP210102924, DP220102812) and supported by an Australian Government Research Training Program (RTP) Scholarship.
Ethics declarations
Competing interests
SWC and AJKP have received research fundings from Delos and Versalux, and they are co-founders of Circadian Health Innovations Pty Ltd. ACL, MTB, BGT and EMM declare no potential conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lander, A.C., Burge, M.T., Thomas, B.G. et al. Circadian photoreception influences loss aversion. Sci Rep 15, 13051 (2025). https://doi.org/10.1038/s41598-025-97370-z
Received: 30 July 2024
Accepted: 03 April 2025
Published: 16 April 2025
DOI: https://doi.org/10.1038/s41598-025-97370-z
.png)