Abstract
Periodontal ligament (PDL) consists mainly of collagen fiber bundles with specialized mechanoreceptors and free nerve endings that connect the cementum of the tooth root to alveolar bone and are vital for dental proprioceptive function. When a tooth is lost and replaced with a dental implant, osseointegration occurs without the intervening PDL, leading to a loss of proprioceptive function. Herein we report the placement, and healthy integration of an advanced dental implant in the socket of rat study models without facilitating the process of osseointegration, that could possibly impart proprioceptive features comparable to those noted in natural teeth. The experimental surgical procedure during dental implant installation in rat models involved various oral tissue structures surrounding the teeth as analogous to those in human subjects and therefore bears a significant clinical relevance. Additionally, the surgical procedure detailed here confers the advantages of its use to investigate not only dental implants but also could be explorative for a wide range of extra-oral implants for improved neural integration.
Similar content being viewed by others
Introduction
Partial or complete edentulism is the state of being edentulous i.e. without one or all natural teeth, respectively. Tooth loss, predominantly, occurs due to consequences of dental diseases, such as dental caries or periodontitis1which may lead to imbalance in the stomatognathic system2and initiating a multitude of extreme ramifications2. It is noteworthy that ageing alone has a minor impact on the ability of patients to reduce food into smaller particles, if the confounding factor of tooth loss is controlled for3. Furthermore, food stiffness/rigidity is also perceived through the periodontal proprioceptive feature of a healthy tooth relaying sensory information to mesencephalic trigeminal nucleus4,5,6 in the midbrain (Fig. 1) during mastication and affects the masticatory force, activity of oral musculature, and mandibular movements all contributing to rhythmic jaw movements, and mashing of food between the teeth3,7. Later, the muscle of mastication are inhibited by sensory receptors in the oropharynx through food bolus and initiates the complicated process of swallowing8,9,10where pharynx is transformed into a tract for food propulsion, momentarily11. Thus, periodontal proprioception along with the neuro-muscular control of chewing contributes to comminution of the food3,7and its propulsion12towards alimentary canal13. In cases of edentulism, prosthodontic management through dental implants, secured through osseointegration are recommended due to numerous advantages14with an estimated projection of its use in roughly 23% of US population by 202615and an increasing trend in dental implants market share of approx. USD 13.01 billion globally16.
However, dental implant replacing a lost tooth bears a substantial physiological consequence due to absence of intra-dental and periodontal mechanoreceptors that alters the precise coordination of maxillo-mandibular relation and impacts the discriminatory, directional and masticatory sensation17. Although, patients rehabilitated with dental implants have shown enhanced tactile discriminative capabilities & motor function compared to complete denture patients18, they do not adequately restore all the oral function to the former stomatognathic privileges19. Scientific evidences concludes that the dental implant osseointegration resulting from secondary stability, lacks the specialized periodontium at the interface and its associated functionality such as a very subtle tactile sensitivity20i.e. periodontal proprioception, leading to altering the precise coordination of oral, pharyngeal, and laryngeal structures17; further deviating from the ideal norm of subsequential digestion-assimilation process indicating towards an largely uncharted “missing of periodontal sensory perception” induced impact on overall health and well-being in humans13,21,22,23.
Hence, to address the limitations of current dental implants19,24,25,26,27,28,29,30 we designed an advanced prototype for dental implants ((Supplementary Methods, Supplementary Fig. 1–8, Supplementary Table 1) specific for inducing proprioceptive features (Fig. 1) and were successful in accurately installing (Fig. 2) and integrating them (Fig. 3) in the tooth socket of rat study models. While confirmatory proprioceptive status will be examined in animals with the specific procedures in the future, we anticipate that the simple surgery and bioengineering strategy implemented in our initial trial evaluations could drive progress in creating new design concepts for the modified dental implant integration that doesn’t involve the conventional osseointegration process. Additionally, the procedural details here will also provide insight into modifying a wide range of other neuro-prosthetics and nerve rehabilitation devices in the direction of superior neurophysiological integration.
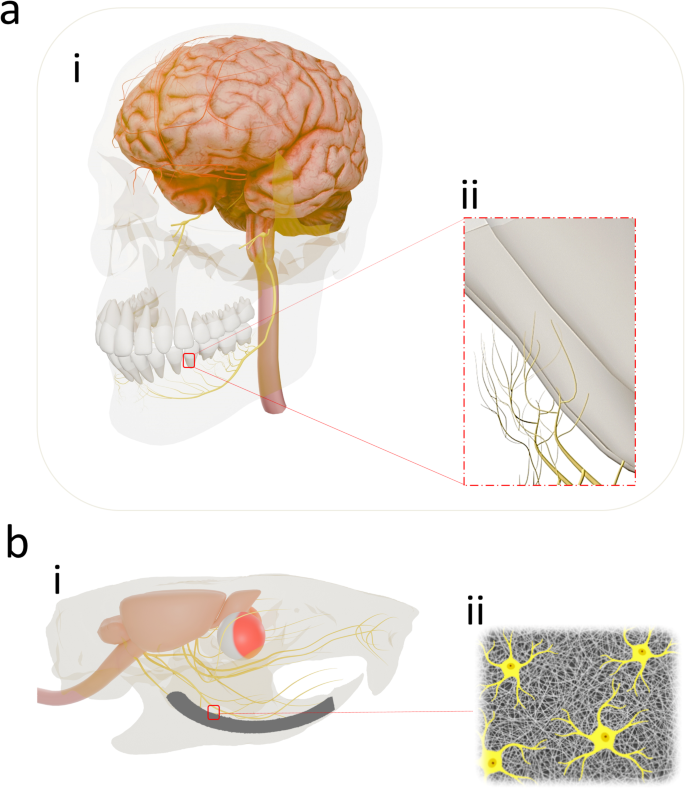
Translational implication of the trial investigation: (a) i, The human brain with the maxillary and mandibular teeth are connected through the trigeminal nerve. For reasons of simplification, only the inferior alveolar nerve arising from the mandibular branch of the trigeminal nerve (CN V3) providing sensory innervation to the mandibular teeth, gingiva, and dental sockets is shown. (a) ii, Besides dental pulp innervation, the roots of the mandibular teeth are covered by the free nerve terminals mostly from the inferior or mental division of the mandibular branch of the trigeminal nerve. (b) i, Through an experimental surgical protocol, the left mandibular central incisor in the rat study models were extracted and subsequently the elastomeric nanofibre coated dental implant with stem cells were placed in the dental socket. Accordingly, appropriate cues were engineered for guiding the stem cell’s fate and differentiation towards neural cells on the biodegradable coating (b)iii influencing a modified integration where the differentiated neural cells present in the regenerated neo-tissue complex may anastomose with the severed/terminal sensory branches in the interior wall of the dental socket arising from the trigeminal nerve.
Methods
Dental implant prototype
The test implants were fabricated with a shorter dimension in comparison to the length of original tooth for sufficient interocclusal space and were later coated with nanofibers (Supplementary Methods). The Growth factor was adsorbed during the coating onto the surface intermittently, proving a multi-layer of orderly pile stack of growth factor adsorbed layers (Supplementary Methods). The undifferentiated immortalized stem cells (Supplementary Methods) were seeded on the surface of the coating with increased concentration of cells in the middle 3rd, lingually.
Animals
Male Brown Norway rats (n = 3 + 3) aged 12 weeks, weighing ~ 225–245 gms upon initiation of the study were purchased from Charles River Breeding labs. Rats were housed with food and water provided ad libitum, light in 14:10 h light: dark cycles, and housed at ambient 22 °C (± 1 °C) temperature with 30–70% humidity.
After a 3-day acclimatization period, the rats were assessed and found to be free of clinical signs of diseases, with normal posture, movement, respiration cycle, cardiovascular status etc. For comparative evaluation hematological, cytokines parameters, temperature, weight and H&E-stained sections of left submandibular cervical lymph node of representative rat models (with and without implants) were obtained at the end of the trail period (Supplementary Fig. 9.) (Supplementary Tables 2–5.). At the end of the trial period the rats were euthanized using carbon dioxide (CO2) inhalation to induce asphyxiation, followed by cervical dislocation as a secondary euthanasia.
Implant surgery
Animals (n = 3) were briefly anaesthetized with 70 mg kg−1 ketamine and 10 mg kg−1 Xylazine. Povidone-iodine swab sticks (Medline, MDS 093902) and 2% chlorhexidine were used to disinfect the intraoral region. Pre-surgical evaluations were meticulously performed for the left mandibular tooth, its surgical anatomy, the length of the crown and its occlusion, interocclusal relationship, alveolar ridge, potential mandibular implant site, etc. Besides the usual oral surgical instruments, modified surgical blades from the hypodermic needles (Exel Int. 25 G, Ref.26403) were fabricated, by flattening, and sharpening their edges. During the surgery, the sterile modified blades were placed into the gingival sulcus of the mandibular left incisor, distally, with gradual tearing of the distal gingival fibers and advancing apically, extending far down, progressing and severing the deeper attachment fibers. Thereafter, the blade was gently proceeded further, in the periodontal space, moving circumferentially, surrounding the tooth, gradually towards mesio-apical orientation, carefully maneuvering around the external contour of the tooth and completely detaching the mesial attachment fibers loosening the tooth (Fig. 2). The tooth was later grasped with tweezers (Harfington, Uxcell, Ref:1174861) gently luxated and extracted. The dental implant coated with immortalized undifferentiated rat dental pulp stem cells (Supplementary Methods) was press fitted and fixed in its natural position in the fresh socket. A cyanoacrylate adhesive dressing (PeriAcryl® 90, GluStitch Inc., Delta, Canada) was used to seal and secure the interface of titanium implants and peri-implant soft tissue from rest of the oral cavity (Fig. 2). Meticulous preoperative and postoperative radiological evaluation (Skyscan 1176 High Resolution Micro-CT Scanner, Bruker) relevant to maxilla-mandibular structures, precise placement of the dental implant and their relation to adjacent structures (Fig. 3) etc. were carried out. For management of postoperative pain, analgesics such as buprenorphine hydrochloride 0.05 mg kg−1 was used.
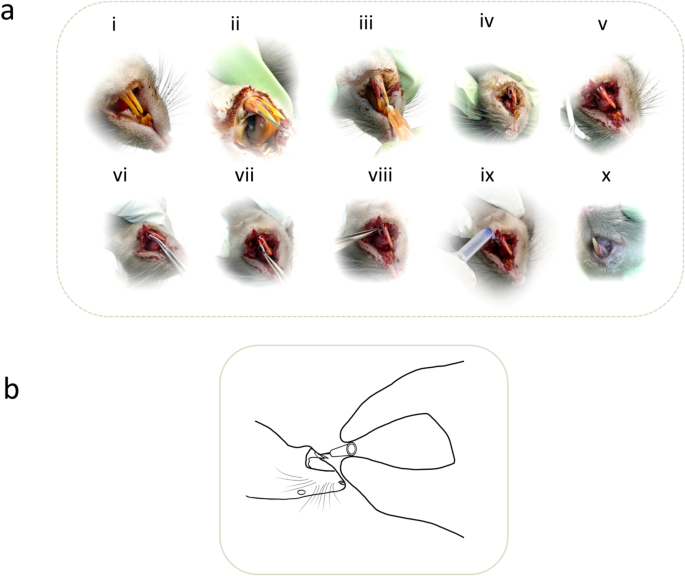
The details of the intraoperative photographs of the representative rat model manifesting the experimental surgical technique for installing titanium dental implant in the mandible. (a) i, Unoperated normal and healthy mandibular central incisors. (a) ii, Careful and gentle insertion of the customized blade into the gingival sulcus distally, gradually advancing towards the center of the tooth and progressively severing the attachment fibres towards the apical orientation. (a)iii, Gentle insertion of the customized blade into the mesial gingival sulcus, and later advancing into the periodontal space with the tip of the blade angled towards the long axis of the incisor and severing the respective attachment fibres around the entire tooth circumference. (a) iv, The structural integrity of the tooth socket is well maintained after gently extracting the loose tooth. (a)v-viii, Elastomeric nanofibrous coated dental implant with stem cells is press-fitted in the dental socket. (a) ix, A tissue adhesive was used to cover the peri-implant region. (A)x, Peri-implant tissue healing after 48 h. (b) Gentle holding of the surgical blade during the experimental surgery ensuring the least traumatic way to divide the tissues.
Post-operative care and housing
Animals were housed individually and during post-anesthesia warming phase, a heating pad, set at ~ 37 °C, was placed beneath each recovery cage. During the post-surgery recovery period, all the animals have 14 h:10 h light/dark cycle and had access to gel diet (DietGel® Recovery & DietGel 76 A, Clear H2O) along with standard food and water ad libitum.
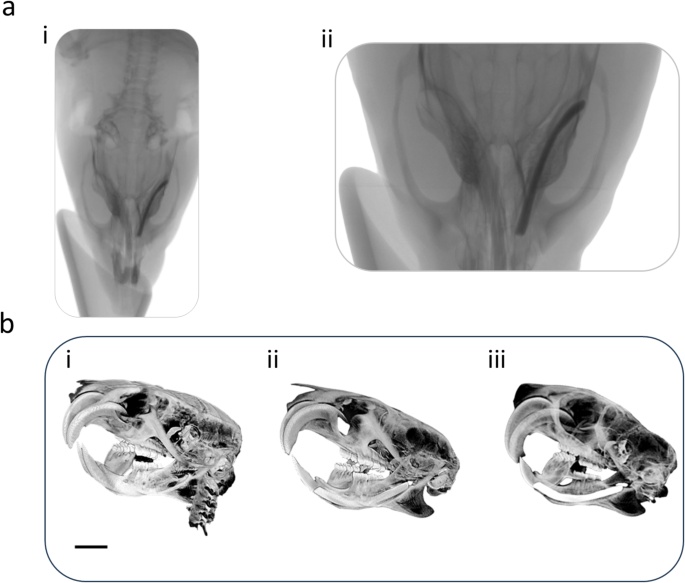
Radiological evaluation. (a)i-ii, Plain radiographs of dental implant after six weeks of dental implant surgery. (b) i Micro-CT images of rats with no dental implants., (b)ii, immediately after placing the implant, and (b) iii, after six weeks post-surgery. Distinct peri-implant radiolucency with no appreciable bone-to-implant contact is noted for both plain radiographs and micro-CT images. Image scale bar in (b) represents 10 mm.
We confirm that the animal experiments were executed in conformity with the relevant guidelines and regulations established by the National Institutes of Health and institutional guidelines with the approval of Tufts University’s IACUC (Protocol #B2020-156).
Results
All the animals survived the whole experimental time, and no operative or postoperative complications were encountered. The clinical and macroscopic evaluation revealed that all the prototypes for dental implants were well fixed in their respective dental sockets at the end of the trial period (Supplementary Video). All the rats presented healed peri-implant mucosa with no evidence of exudation, inflammation or crusting. Micro-CT investigation after 6 weeks post-implantation revealed distinct peri-implant radiolucency as a narrow radiolucent space in the range of 0.7–0.9 mm between the alveolar bone and the dental implant indicating absence of osseous/calcified characteristics in the integrating tissue (Fig. 3). Notably, absence of cervical lymphadenopathy throughout the investigation, along with the comparative histological evaluation (Supplementary Fig. 9). of the left submandibular lymph node, blood biochemistry, hemogram, cytokine levels, temperature, weight (Supplementary Tables 2–5) etc. indicated uneventful recovery.
Discussion
As periodontium in the healthy natural tooth are equipped with highly specialized periodontal mechanoreceptors31,32it is unlikely that any regenerative intervention directed towards proprioceptive titanium dental implant will produce a physiologic outcome that is equivalent to that of a healthy and true periodontium. Conversely, some reports also highlight about the presence of periodontal mechanoreceptors even after the tooth extraction33and that had led to investigation of reinnervation in relation to titanium dental implants34 but with no success.
It is largely agreed that the sensory component of the periodontal ligament contains rich sensory non-encapsulated terminal receptors including free nerve endings and specialized Ruffini-like endings35. In humans, the periodontium receives innervating nerve fibers36,37mostly through the apical region and some course through the lateral foramina in the alveolar bone38. Following routine exodontic procedures, the nerve fibers innervating the periodontal ligaments and the pulp are crushed and severed. During the subsequent period of normal socket healing39there is a possibility that these traumatized and residual nerve fibers may regenerate into and through the soft tissue of the socket, innervating the periosteum or alveolar mucosa. Alternatively, they may form a traumatic or amputation neuroma within the jawbone40.
Hence, our proof-of-concept trial deals with targeted repairing of these injured terminal nerve endings instead of the whole periodontium, that are present as an extension of the trigeminal system at the interface of the immediately placed dental implant and alveolar bone through a significantly less traumatic experimental surgical protocol, thereby restoring the neural circuitry in an otherwise intact dental proprioceptive route that communicates from the interface of a tooth root/dental implant to the mesencephalic nucleus in the midbrain.
Consequently, for achieving our objective a modified integration of dental implants is crucial and accordingly the advanced prototype for dental implants were installed based on the press-fit phenomenon41. Additionally, multiple innovative strategies were implemented to engineer the dental implant integration that is different from the usual or conventional “osseointegrative” way; such as coating dental implants with elastomeric biodegradable nanofibers, multilayer incorporation of hyperstable FGF-β in the coating, seeding exogenous rat’s dental pulp stem cells in the coating that were concentrated in the region corresponding to the alveolar half of the lingual periodontal ligament and were based on the neuroanatomical details of the rat’s incisor42,43,44,45,46 etc.
Finally, and above all, the requisite atraumatic tooth extraction were performed on rat study models by using customized flexible & sharp blades. These blades were fabricated by flattening and sharpening the edges of the syringe needles for gently disengaging the periodontium and extracting the complete tooth with least trauma to the peri-dental tissue for maintaining the structural integrity of the socket and preserving the maximal severed periodontium is the interior wall of the socket containing the unencapsulated nervous elements such as Ruffini-like corpuscles and free nerve endings.
Upon completing the immediate dental implant placement in the socket, the deformed and compressed elastomeric nanofibrous coating, slowly resumes its shape conforming the interior dimension of the socket ensuring a snug or tight fit and further resulting the attached exogenous dental pulp stem cells on the coating to directly interface the aforementioned nervous elements. This closely fit status of the dental implant with no appreciable signs of mobility may be comparable to primary stabilityof the conventional titanium dental implants, that are observed and favoured during surgical placement and arises from the mechanical friction between implant surface and the surrounding bone14,47.
Likewise, herein, over a period of time & under the influence of hyper stable fibroblast growth factor 2 (FGF 2)48in the coating, orthotopic environment49,50and experimental surgery, the elastomeric nanofibrous coating with stem cells in the dental socket slowly deteriorates with progressive repairing of the terminal nerve endings48 including possible neural anastomosis and gradually replacing the interfacial space occupied by the elastomeric coating with neo-tissues bridging alveolar bone and implant surface having features dissimilar to osseous tissue yet could be analogous to well-established secondary stability14,47, in the healing stages that completes the osseointegration process, noted in the conventional dental implants. Convincingly, the 6 weeks old integrated prototypes were neither tender to percussion or pressure nor was mobile (Supplementary video)51, together with the distinct per-implant radiolucency (Fig. 3), the occurrence of the purely fibrous integration52or fibro-osseous integration53 were therefore excluded.
Although this innovate surgical trial was performed on small sample size of preclinical study models, the surgical anatomy of peri-dental region specifically the minimally invasive experimental surgical technique limited only to connective tissue attachment fibers such as periodontium, marginal gingiva, attached gingiva, etc. and the modifications in the prototype of titanium dental implants, bears a close resemblance to actual cases managed in clinical scenario and indicates that the modified integration of the prototype dental implants towards proprioceptive function could be a formidable alternative to its well established oseeointegrative counterpart. However, such engineered integration results in dental implant to be proprioceptive needs to be confirmed especially through advanced neurodiagnostic imaging techniques for prompting a clinical trial.
Advantageously, reports in the literature also explains about the rodents such as mole rats where extraordinary brain organization i.e. nearly one-third (31%) of primary somatosensory cortex is devoted to the representations of the upper and lower incisors54. Therefore, assuming homology of the neuro-anatomical organization across species with aradicular hypsodonty in terms of both somatosensory representation and the resulting functional connectivity, the similar prototype implants for mandibular incisors with the exact surgical procedures described here will be investigated for proprioceptive function on Brown Norway rats (Rattus norvegicus) in the next phase of the trial.
Limitations
Variations in technique among surgeons performing the same procedure can influence the consistency of outcomes.
Data availability
All data supporting the findings of this study are included within this published article and its supplementary materials.
References
Rozier, R. G., White, B. A. & Slade, G. D. Trends in oral diseases in the U.S. Population. J. Dent. Educ. 81, (2017).
Rosenstiel, S. F. & Land, M. F. Contemporary Fixed Prosthodontics-E-Book: Contemporary Fixed Prosthodontics-E-Book (Elsevier Health Sciences, 2015).
Woda, A., Mishellany, A. & Peyron, M. The regulation of masticatory function and food bolus formation. J. Oral Rehabil. 33, 840–849 (2006).
Dhar, A. et al. The periodontium damage induces neuronal cell death in the trigeminal mesencephalic nucleus and neurodegeneration in the trigeminal motor nucleus in C57bl/6J mice. Acta Histochem. Cytochem. 54, 11–19 (2021).
Tal, M. & Devor, M. Anatomy and neurophysiology of orofacial pain. Orofac. Pain Headache Edinb. Elsevier 19–44 (2008).
Jerge, C. R. Organization and function of the trigeminal mesencephalic nucleus. J. Neurophysiol. 26, 379–392 (1963).
van der Bilt, A., Engelen, L., Pereira, L. J., Van der Glas, H. W. & Abbink, J. H. Oral physiology and mastication. Physiol. Behav. 89, 22–27 (2006).
Capra, N. F. Mechanisms of oral sensation. Dysphagia 10, 235–247 (1995).
Washabau, R. J. & Day, M. J. Canine and Feline Gastroenterology (Elsevier Health Sciences, 2012).
Takeda, H. & Saitoh, K. Impact of proprioception during the oral phase on initiating the swallowing reflex: phase transition during swallowing. Laryngoscope 126, 1595–1599 (2016).
Hall, J. E. & Hall, M. E. Guyton and Hall Textbook of Medical Physiology E-Book: Guyton and Hall Textbook of Medical Physiology E-Book (Elsevier Health Sciences, 2020).
Peyron, M. A. et al. Role of physical bolus properties as sensory inputs in the trigger of swallowing. PloS One. 6, e21167 (2011).
Palmer, C. A. Age-Related changes in oral health status: effects on diet and nutrition. In Nutrition and Oral Medicine (eds Touger-Decker, R. et al.) 31–43 (Humana, 2005). https://doi.org/10.1385/1-59259-831-5:031.
Misch, C. E. Dental Implant Prosthetics-E-Book (Elsevier Health Sciences, 2004).
Elani, H. W., Starr, J. R., Silva, D., Gallucci, G. O. & J. D. & Trends in dental implant use in the U.S., 1999–2016, and projections to 2026. J. Dent. Res. 97, 1424–1430 (2018).
Saghiri, M. A., Freag, P., Fakhrzadeh, A., Saghiri, A. M. & Eid, J. Current technology for identifying dental implants: a narrative review. Bull. Natl. Res. Cent. 45, 7 (2021).
Klineberg, I., Murray, G. & Osseoperception Sensory function and proprioception. Adv. Dent. Res. 13, 120–129 (1999).
González-Gil, D., Flores-Fraile, J. & López-Marcos, J. Tactile sensibility thresholds in implant prosthesis, complete dentures and natural dentition: review about their value in literature. Med. (Mex). 58, 501 (2022).
Higaki, N. et al. Do sensation differences exist between dental implants and natural teeth? A meta-analysis. Clin. Oral Implants Res. 25, 1307–1310 (2014).
Hartmann, F. & Cucchi, G. Stress and Orality: New Data about Teeth Clenching & Outcomes, Migraine, Fibromyalgia, Fatigue (Springer Paris, 2014). https://doi.org/10.1007/978-2-8178-0271-8
Ship, J. A., Duffy, V., Jones, J. A. & Langmore, S. Geriatric oral health and its impact on eating. J. Am. Geriatr. Soc. 44, 456–464 (1996).
Budtz-Jørgensen, E., Chung, J. P. & Rapin, C. H. Nutrition and oral health. Best Pract. Res. Clin. Gastroenterol. 15, 885–896 (2001).
Tosello, A. et al. Oral functional characteristics and Gastrointestinal pathology: an epidemiological approach. J. Oral Rehabil. 28, 668–672 (2001).
Hsieh, W. W., Luke, A., Alster, J. & Weiner, S. Sensory discrimination of teeth and Implant-Supported restorations. Int. J. Oral Maxillofac. Implants 25, (2010).
Meyer, G., Fanghänel, J. & Proff, P. Morphofunctional aspects of dental implants. Ann. Anat. -Anat Anz. 194, 190–194 (2012).
Jacobs, R. & Van Steenberghe, D. From osseoperception to implant-mediated sensory‐motor interactions and related clinical implications*. J. Oral Rehabil. 33, 282–292 (2006).
Koyano, K. & Esaki, D. Occlusion on oral implants: current clinical guidelines. J. Oral Rehabil. 42, 153–161 (2015).
Hämmerle, C. H. F. et al. Threshold of tactile sensitivity perceived with dental endosseous implants and natural teeth. Clin. Oral Implants Res. 6, 83–90 (1995).
Lobbezoo, F., Brouwers, J. E. I. G., Cune, M. S. & Naeije, M. Dental implants in patients with Bruxing habits. J. Oral Rehabil. 33, 152–159 (2006).
Stanford, C. M. & Schneider, G. B. Functional behaviour of bone around dental implants*. Gerodontology 21, 71–77 (2004).
Trulsson, M. Sensory-motor function of human periodontal mechanoreceptors*. J. Oral Rehabil. 33, 262–273 (2006).
Hihara, H. et al. Somatosensory evoked magnetic fields of periodontal mechanoreceptors. Heliyon 6, (2020).
Linden, R. W. A. & Scott, B. J. J. The effect of tooth extraction on periodontal ligament mechanoreceptors represented in the mesencephalic nucleus of the Cat. Arch. Oral Biol. 34, 937–941 (1989).
Bonte, B., Linden, R. W., Scott, B. J. & Van Steenberghe, D. Role of periodontal mechanoreceptors in evoking reflexes in the jaw-closing muscles of the Cat. J. Physiol. 465, 581–594 (1993).
Linden, R. W. Periodontal mechanoreceptors and their functions. Neurophysiol. Jaws Teeth 52–95 (1990).
Lambrichts, I., Creemers, J. & Van Steenberghe, D. Morphology of neural endings in the human periodontal ligament: an electron microscopic study. J. Periodontal Res. 27, 191–196 (1992).
Willis, R. D. & DiCosimo, C. J. The absence of proprioceptive nerve endings in the human periodontal ligament: the role of periodontal mechanoreceptors in the reflex control of mastication. Oral Surg. Oral Med. Oral Pathol. 48, 108–115 (1979).
Hildebrand, C., Fried, K., Tuisku, F. & Johansson, C. S. Teeth and tooth nerves. Prog Neurobiol. 45, 165–222 (1995).
Steiner, G. G. et al. The healing socket and socket regeneration. Compend Contin Educ. Dent. 29, 114–116 (2008).
Hansen, H. J. Neuro-histological reactions following tooth extractions. Int. J. Oral Surg. 9, 411–426 (1980).
Golec, T. S. Technique for press-fit implants. J. Am. Dent. Assoc. 1939. 121, 409–412 (1990).
Sato, O. et al. Innervation of periodontal ligament and dental pulp in the rat incisor: an immunohistochemical investigation of neurofilament protein and glia-specific S-100 protein. Cell. Tissue Res. 251, 13–21 (1988).
Kakuta, W. et al. Regeneration of sensory nerve branches in extraction socket and surrounding alveolar bone in rat: immunohistochemical observation of the axon and Myelin sheath changes. Odontology 111, 630–639 (2023).
Imai, T., Atsumi, Y., Matsumoto, K., Yura, Y. & Wakisaka, S. Regeneration of periodontal ruffini endings of rat lower incisors following nerve cross-anastomosis with mental nerve. Brain Res. 992, 20–29 (2003).
Magaudda, L. et al. Nerve endings in rat periodontal ligament: an Immunofluorescence study. Ital. J. Anat. Embryol. 121–121 (2016).
Muramoto, T., TAKANO, Y. & Soma, K. Time-related changes in periodontal mechanoreceptors in rat molars after the loss of occlusal stimuli. Arch. Histol. Cytol. 63, 369–380 (2000).
Misch, C. E. & Resnik, R. Misch’s Avoiding Complications in Oral Implantology (Elsevier Health Sciences, 2017).
Zheng, K. et al. Basic fibroblast growth factor promotes human dental pulp stem cells cultured in 3D porous Chitosan scaffolds to neural differentiation. Int. J. Neurosci. 131, 625–633 (2021).
Tanaka, J. et al. Generation of orthotopically functional salivary gland from embryonic stem cells. Nat. Commun. 9, 4216 (2018).
Mitsiadis, T. A., Barrandon, O., Rochat, A., Barrandon, Y. & De Bari, C. Stem cell niches in mammals. Exp. Cell. Res. 313, 3377–3385 (2007).
Duyck, J. & Naert, I. Failure of oral implants: aetiology, symptoms and influencing factors. Clin. Oral Investig. 2, 102–114 (1998).
Linkow, L. I., Giauque, F., Ghalili, R. & Ghalili, M. Levels of osseointegration of blade-/plate-form implants. J. Oral Implantol. 21, 23–34 (1995).
Mavrogenis, A. F., Dimitriou, R., Parvizi, J. & Babis, G. C. Biology of implant osseointegration. J. Musculoskelet. Neuronal Interact. 9, 61–71 (2009).
Catania, K. C. & Remple, M. S. Somatosensory cortex dominated by the representation of teeth in the naked mole-rat brain. Proc. Natl. Acad. Sci. 99, 5692–5697 (2002).
Acknowledgements
The authors would like to express sincere gratitude to the Bray Lab and Nano Lab, at Tufts University School of Engineering and the George J. Kostas Research Institute for Homeland Security for providing access to advanced facilities that were essential to the completion of this research project.
Funding
The authors would like to acknowledge the support from the National Institutes of Health (NIH) with grants DK131444, DE30074, DE25681 and DE32006 to Jake Chen. This study was conducted in accordance with the Declaration of Helsinki and Tufts University policies.
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All animal procedures were compliant as per the relevant ethics regulations established by the National Institutes of Health and institutional guidelines with the approval of Tufts University’s IACUC (Protocol #B2020-156). All animal procedures were conducted in accordance with the ARRIVE guidelines.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Das, S., Ghosh, S., Tu, Q. et al. Surgical considerations towards inducing proprioceptive feedback in dental implants. Sci Rep 15, 15208 (2025). https://doi.org/10.1038/s41598-025-99923-8
Received: 23 October 2024
Accepted: 23 April 2025
Published: 30 April 2025
DOI: https://doi.org/10.1038/s41598-025-99923-8
.png)