Introduction
Tropism is the ability of immobile plants to place leaves and other organs at appropriate locations by adjusting the direction of growth. The two main types of tropism are gravitropism and phototropism1,2. Negative and positive gravitropism allows plants to grow shoots and roots upward and downward, respectively2,3. Phototropism maximises photosynthetic production under natural conditions by avoiding shade and competing with other plants4. Generally, both gravitropism and phototropism are essential for successful growth and reproduction by exposing plant organs to the required resources, that is, leaves to light, roots to moisture and nutrients, and flowers to pollinators1,4. Flowers have antagonistic requirements for reproductive success depending on the time of day, environmental conditions, and weather; that is, attracting pollinators in the sun and also protecting flowers in the rain and at night. Gravity signals are characterised by their permanence and fixed direction, whereas light signals are often transient and vary in strength and direction. Therefore, whether gravitropism or phototropism predominates can change depending on the weather conditions, and such a switch will allow flowers to meet these two antagonistic demands. However, crosstalk between gravitropism and phototropism has not yet been addressed.
In flower stems, gravitropism and phototropism play key roles in attracting pollinators by adjusting the flower orientation. For example, in bilaterally symmetrical flowers, flower orientation relative to gravity determines the positional fit between pollinator bodies and floral organs5,6. Heliotropism is a well-known light-induced tropic response of flower orientation, in which flowers track the sun7,8. Studies on sunflowers have shown that heliotropism is caused by differential elongation on opposite sides of the stems of immature plants, induced by asymmetric auxin regulation that depends on circadian-gated tropism and autostraightening9,10. Heliotropism results in higher flower surface temperatures that attract more pollinators8,11,12. The role of flower orientation has been studied mainly in relation to pollinator attraction11,12. However, pollination success is not the only factor that affects reproductive success.
The protection of flowers is also an important factor for reproductive success, as floral organs are generally susceptible to environmental stresses, such as raindrops, UV radiation, frost and heat13,14,15. Flower orientation may provide protection, and several studies have reported that horizontal and downward orientations prevent water damage to pollen during rainfall16,17. The activities of insect pollinators are sensitive to weather conditions, and they are strongly suppressed in rain, which is often associated with low temperature and low sunshine18,19,20; in such circumstances, flowers may change their orientation to be more protective. However, whether plant tropism enhances flower protection depending on weather conditions remains unclear. Plants may possess sophisticated tropism control to simultaneously satisfy pollinator attraction and flower protection by changing flower orientation depending on the weather.
In this study, we discovered that flowers of Arabidopsis halleri face upward in the sun and downward in the rain by moving their flower pedicels in a weather-dependent fashion, that is, weather-driven flower movement (Fig. 1a). We hypothesised that flowers would attract more pollinators by facing upward in the sun, while avoiding flower damage by facing downward in the rain and at night when pollinator activity is minimal, thereby increasing plant reproductive success. We further predicted that weather-driven flower movement is caused by a combination of gravitropism and phototropism of flower pedicels, which are under circadian regulation. To test the hypotheses and predictions, we conducted the following analyses: (1) field observations of flower angles and pollinator activities, (2) experiments to identify the type of tropism that causes weather-driven flower movements, (3) gene expression analyses, and (4) experiments to test the adaptive significance of weather-driven flower movements. A combined study of field observations, experiments, and transcriptomics under natural and controlled conditions enabled us to elucidate how different tropisms are coordinated under fluctuating weather conditions to achieve antagonistic requirements for flowers, that is, attraction and protection.
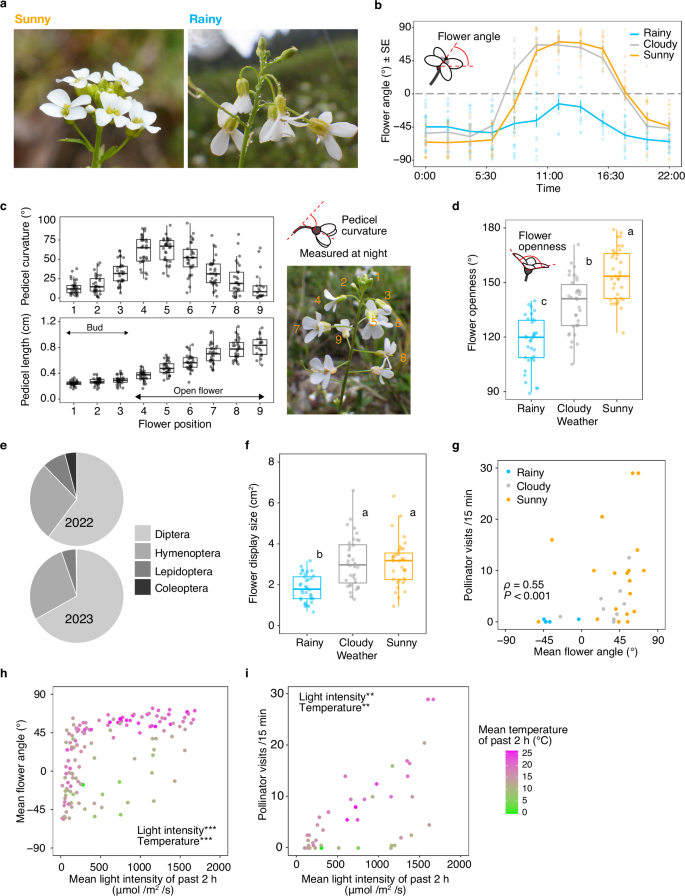
a Flowers on a sunny day (left) and on a rainy day (right). b Diel changes in flower angle under different weather conditions. Values are means ± SE. n = 13–24 pedicels from 7 to 8 plants for each weather condition (precise n for each time point is listed in Source Data file). c Pedicel curvature and length along flower stages from flower buds to wilting flowers (n = 30). d Flower openness under different weather conditions (n = 34). e Pollinator composition in 2022 and 2023. f Flower area per inflorescence projected in a vertical direction under different weather conditions (n = 36). g Relationship between flower angles and frequency of pollinator visits (number per 15 min) under different weather conditions. h Dependency of flower angles on light intensity (photosynthetic photon flux density) and temperature during the past 2 h. i Dependency of pollinator visits on light intensity and temperature during the past 2 h. In the box plots (c, d, and f), 25%, 50%, and 75% of the data are indicated using hinges and centre lines. The whiskers extend from the hinges to the highest and lowest values, which are within 1.5× of the inter-quartile ranges. In (d) and (f), different letters indicate significantly different flower openness and display size in pairwise comparisons (two-sided; p < 0.05). In g, Spearman rank correlation coefficient (ρ) and its significance level are indicated (two-sided). In (h) and (i), asterisks indicate two-sided p values, ***p < 0.001and ** p < 0.01. Source data are provided as a Source Data file.
Results
Environmental cues affecting weather-dependent flower movement and pollinator activities
Monitoring the flower angle to the horizontal using interval cameras revealed that flowers face downward at night and on rainy days (Fig. 1b). On sunny and cloudy days, flowers started to turn upward early in the morning, reached maximum levels by 10:00, and then started to turn downward at 16:00 (Fig. 1b). We determined the flowering stages at which the pedicels could bend. The curvature and length of the pedicels at each flower position were measured at night when the pedicels bent the most (Fig. 1c). The degree of pedicel curvature was low in the early stages of flower buds, started to increase at the bud stage prior to opening, reached a maximum in the early stages of open flowers, and declined gradually towards the late stages of open flowers (Fig. 1c). Pedicel elongation was also observed during flower opening (Fig. 1c), and we observed an increase in the length of the epidermal cells of the pedicel corresponding to this period (Supplementary Fig. 1). Therefore, the pedicel bends specifically during pedicel elongation at the open flower stage. Flowers opened the most on sunny days, slightly less on cloudy days, and even less on rainy days; these differences were statistically significant (p < 0.0001) (Fig. 1d and Supplementary Table 1). However, even on rainy days, the degree of flower closure was low, averaging 117° open (Fig. 1d).
During the two-year observation period, flowers were visited by various insect pollinators, including those that preferentially visited upward-facing flowers, such as Diptera and Coleoptera (Fig. 1e, Supplementary Fig. 2a and b, and Supplementary Table 2). The flower display size per inflorescence (vertical view) measured at noon was larger on sunny and cloudy days than on rainy days (p < 0.0001) (Fig. 1f and Supplementary Table 1), indicating that upward-facing flowers on sunny and cloudy days were more visible to pollinators. A positive correlation was detected between the flower angle and frequency of pollinator visits (Spearman rank correlation coefficient (ρ) = 0.55, p = 0.0010) (Fig. 1g and Supplementary Fig. 3a). Light intensity increased towards noon and decreased towards dusk on sunny and cloudy days (Supplementary Fig. 3b). The light spectrum diagrams showed similar light quality patterns on both rainy and sunny days (Supplementary Fig. 3c). Daytime records for 15 d indicated that both the flower angle and frequency of pollinator visits increased with the mean light intensity (flower angle, p < 0.0001; pollinator visits, p = 0.0091) and temperature (flower angle, p < 0.0001; pollinator visits, p = 0.0021) during the past 2 h (Fig. 1h, i, and Supplementary Tables 3 and 4). The flower angle and mean humidity during the past 2 h showed a negative relationship (p < 0.0001; Supplementary Fig. 3d and Supplementary Table 4). As light intensity, temperature, and humidity are highly correlated with each other, manipulation experiments are required to determine a causal relationship.
Effects of circadian clock, blue light, temperature, and humidity on the flower movement
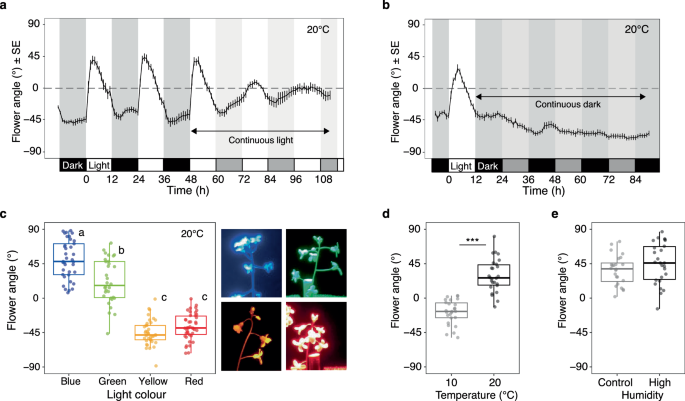
We investigated flower movement in a growth chamber to determine the external and internal factors affecting flower movement. During the 12-h light/dark (LD) cycles, the flowers turned rapidly upwards (ca. 45°/h) within 2 h of the lights being switched on and gradually turned downward by the beginning of the dark (Fig. 2a). Under continuous light conditions after the LD cycles, the flowers faced upward during the subjective day, but the rapid response in the morning disappeared (Fig. 2a). Under continuous dark conditions after the LD cycles, the flowers continued to face downward even during the subjective day (Fig. 2b). The free-running rhythm observed only under continuous light suggests that both light and the circadian clock are important for flower movement.
a Changes in flower angles under LD (12-h LD cycles) and subsequent LL (continuous light) conditions. Actual night and subjective night periods are indicated using dark and weak shading, respectively. Values are means ± SE. n = 14 pedicels from 6 plants. b Changes in flower angle under LD (12-h LD cycles) and subsequent DD (continuous dark) conditions. During the continuous dark, subjective night and day are indicated using dark and weak shading, respectively. Values are means ± SE. n = 18 pedicels from 6 plants. c Flower angles under different light colours (peak wavelengths are 460, 516, 600 and 630 nm for blue, green, yellow, and red, respectively). n = 32–42 pedicels from 11 to 14 plants (precise n for each time point is listed in Source Data file). Different letters indicate significantly different flower angle in pairwise comparisons (two-sided; p < 0.05). d Flower angles under different temperature conditions (10 °C and 20 °C). n = 26 pedicels from ten plants. Asterisks indicate two-sided p values, ***p < 0.001. e Flower angles under control and high humidity conditions (~80% and 99% humidity, respectively). n = 24 pedicels from 7 plants. In the box plots (c–e), 25%, 50%, and 75% of the data are indicated using hinges and centre lines. Source data are provided as a Source Data file.
Light intensity under continuous light conditions was not found to affect the period length of the circadian rhythm, confirming the influence of the circadian clock on flower movements (Supplementary Fig. 4). However, light intensity altered the range and phase of the response, with flowers turning upward more strongly and for longer periods of time under higher light conditions (Supplementary Fig. 4). The flowers responded most strongly to blue light (460 nm), followed by green light (516 nm), but not to yellow (600 nm) or red (630 nm) light (p < 0.0001) (Fig. 2c and Supplementary Table 1). Temperature manipulation experiments revealed that the flower upward-facing requires a warm temperature (20 °C) (p < 0.0001) (Fig. 2d and Supplementary Table 1). High humidity did not disturb the flower upward-facing (p = 0.1161) (Fig. 2e and Supplementary Table 1).
Pedicel-specific upregulated genes during the flower movement
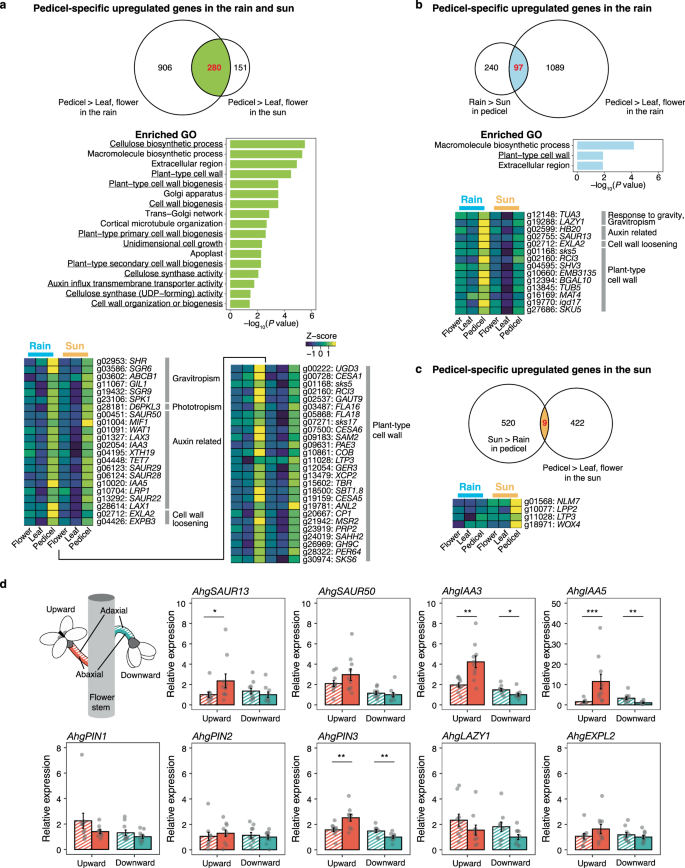
We analysed the transcriptomes of the pedicels, leaves, and flowers in rain and sun to identify genes that were upregulated specifically in the pedicels of downward- and upward-facing flowers in rain and sun, respectively. Samples were collected from five individuals in the natural population for each weather condition between 14:30 and 15:30. In rain and sun, 1186 and 431 genes, respectively, were upregulated in pedicels compared to leaves and flowers (Supplementary Fig. 5 and Supplementary Data 1 and 2). Among these genes, we selected 280 upregulated genes in pedicels, commonly in rain and sun, from the overlap between the selected 1186 and 431 genes (Fig. 3a and Supplementary Data 3). Furthermore, 97 genes showed higher expression in the rain than in the sun (pedicle-specific upregulated genes only in the rain; Fig. 3b and Supplementary Data 4), and vice versa (9 genes only in the sun; Fig. 3c and Supplementary Data 5). The corresponding enriched GO terms for pedicel-specific upregulated genes commonly for both weather conditions, only in the rain and only in the sun, were 17, 3, and 0, respectively (Fig. 3a–c and Supplementary Tables 5 and 6). Enriched GOs in the pedicel under both weather conditions included multiple GOs related to cell wall and cellulose biosynthesis and auxin influx transmembrane transporter activity, suggesting the involvement of active reorganisation of cell walls in response to auxin signals during both upward- and downward-facing movements (Fig. 3a). The enriched GOs in the pedicel in the rain treatment included ‘plant-type cell wall’ and ‘extracellular region’, indicating active reorganisation of the cell walls (Fig. 3b).
a–c Transcriptome analyses of flower, leaf, and pedicel samples in the rain and sun. Pedicel-specific upregulated genes in both weathers (a, top), rain (b, top), and sun (c, top) are identified by overlaps (red numbers) of Venn diagrams. Enriched Gene Ontology (GO) terms in pedicel-specific upregulated genes are shown for those in both weathers (a, centre) and those in the rain (b, centre). No GO were enriched for those in the sun. Enrichment was tested using one-sided Fisher’s exact test. Heatmaps of expression [log2 (rpm+1)] of selected genes for pedicel-specific upregulated genes in both weathers (a, bottom), rain (b, bottom), and sun (c, bottom) are shown. Gene expression in flower, leaf, and pedicel in the rain and sun is shown as Z-scores for each gene. Based on GOs, we listed genes that are classified into ‘gravity’ (GOs with gravitropism and gravity in their names), ‘auxin-related’ (response to auxin and regulation of auxin polar transport), and ‘plant-type cell wall’ (GOs with plant-type cell wall in their names) are shown. All known genes are listed for pedicel-specific upregulated genes in the sun treatment. d RT-qPCR measurements of relative gene expression in adaxial and abaxial halves of the pedicel during upward and downward facing for auxin responsive (AhgSAUR13, AhgSAUR50, AhgIAA3, AhgIAA5), auxin efflux carrier (AhgPIN1, AhgPIN2, AhgPIN3), gravitropism (AhgLAZY1), and cell wall loosening (AhgEXPL2). Values were standardised against an internal control, AhgACT2. Values are shown relative to the average gene expression of the abaxial part when facing downward. Means ± SE of ten biological replicates are shown. Significant differences between adaxial and abaxial parts are indicated using asterisks (***p < 0.001; **p < 0.01; and *p < 0.05 with two-sided Wilcoxon signed-rank sum test). Source data are provided as a Source Data file.
Many genes related to gravitropism, phototropism, auxin, and plant-type cell walls were upregulated pedicel-specifically both in rain and sun, indicating that downward-facing and upward-facing movements share a considerable part of their mechanisms, which involve pedicel elongation via auxin response and cell-wall reconstruction (Fig. 3a). Among these, SHORT ROOT (SHR), SHOOT GRAVITROPISM 6 (SGR6), SGR9, and SPIKE1 (SPK1) are essential for gravitropic response21,22,23,24. SHR is associated with the differentiation or development of amyloplasts in shoot statocytes22. SGR6 is involved in vacuolar membrane dynamics in gravity-sensing cells21, and SGR9 promotes the detachment of amyloplasts from actin filaments23. Other examples are the four SMALL AUXIN-UPREGULATED RNA (SAUR) and two INDOLE-3-ACETIC ACID (IAA) genes, some of which are indispensable for the response to asymmetric auxin distribution during stem elongation25,26,27,28.
We searched for genes that were upregulated only in rain among those highly expressed in pedicels, as these genes are expected to function when the pedicel is bent downward. We identified genes related to gravitropism, auxin, and plant-type cell wall, including LAZY1, SAUR13, and EXPANSIN LIKE A2 (EXLA2)25,29 (Fig. 3b). LAZY1 is thought to function in gravity signalling after amyloplast displacement in statocytes25. These results supported the idea that the downward-facing of flowers involves the gravitropic response of the pedicel. No genes related to phototropism or gravitropism were included in the nine genes that were upregulated pedicel-specifically only under sunny conditions (Fig. 3c).
We hypothesised that asymmetric auxin distribution between the adaxial and abaxial sides of the pedicel caused weather-driven flower movement. We then compared the expression of selected genes associated with the auxin response, gravitropism, and cell wall loosening between the adaxial and abaxial halves of the pedicel of the upward- and downward-facing flowers in the growth chamber using quantitative PCR with reverse transcription (RT-qPCR; Fig. 3d). The pedicel was collected at a single time point when it was bent, 30 min after turning the light on or off. Two auxin-responsive genes, AhgIAA3 and AhgIAA5, and the auxin efflux carrier AhgPIN3, were reciprocally higher in the abaxial and adaxial halves of the pedicels for upward- and downward-facing flowers, respectively (AhgIAA3: p = 0.0021 and 0.0115, AhgIAA5: p = 0.0001 and 0.0011, and AhgPIN3: p = 0.0085 and 0.0067 for upward- and downward-facing flowers, respectively), suggesting that auxin responses were stronger in the outer half of the bending pedicel via directional transport of auxin (Fig. 3d). AhgSAUR13, AhgSAUR50, AhgPIN2, and AhgEXPL2 showed similar tendencies, although a significant difference was found only in AhgSAUR13 for upward-facing flowers (AhgSAUR13: p = 0.0355 and 0.1717; AhgSAUR50: p = 0.4693 and 0.3527; and AhgPIN2: p = 0.2799 and 0.6696 for upward- and downward-facing flowers, respectively) (Fig. 3d). These results indicate that differential auxin signalling between the adaxial and abaxial parts causes flower movement. Previous studies reported that PIN3 is an essential auxin transporter for asymmetric auxin distribution during both shoot gravitropic and phototropic responses30,31. Furthermore, IAA5 is considered an indicator of the gravitropic response because IAA5 expression in A. thaliana inflorescences is markedly increased at the elongating side of the stem in response to gravistimulation28. Application of N-1-naphthylphthalamic acid (NPA), an inhibitor of PIN function32, inhibited flower movement (Supplementary Fig. 6). These results suggest that PIN-dependent asymmetric auxin distribution in the pedicel causes weather-driven flower movement.
Consequences of flower movement on pollinator attraction and flower protection
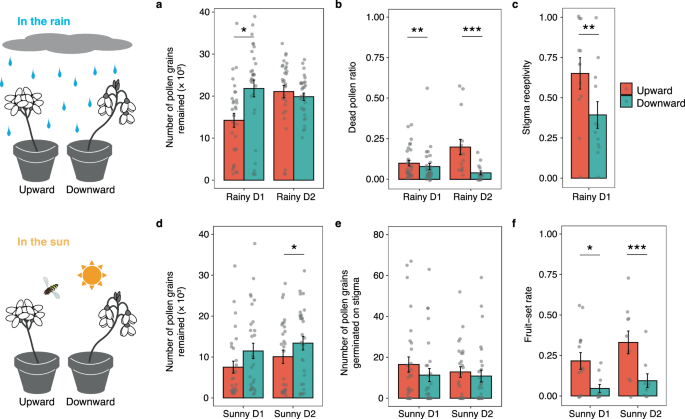
We tested the adaptive significance of weather-driven flower movement using field manipulation experiments. We prepared upward- and downward-facing flowers by placing them under high light and dark conditions for 3 h, respectively, and setting them outside in rain (1 h) or sunlight (2 h). The experiments were conducted on 2 rainy days (Rainy D1 and Rainy D2) and on 2 sunny days (Sunny D1 and Sunny D2). We assessed whether downward-facing functioned to prevent pollen and stigma damage from rainfall. As expected, the number of pollen grains remained on anthers in downward-facing flowers was larger than that in upward-facing flowers on Rainy D1 (p = 0.0277 and 0.6147 in Rainy D1 and D2, respectively) (Fig. 4a and Supplementary Table 7), and the dead pollen ratio in downward-facing flowers was lower than that in upward-facing flowers on both days (p = 0.0039 in Rainy D1 and p < 0.0001 in Rainy D2) (Fig. 4b and Supplementary Table 7), indicating that downward-facing flowers reduce pollen damage from rain. Contrary to our expectations, stigma receptivity was lower in downward-facing flowers than in upward-facing flowers (p = 0.0022) (Fig. 4c and Supplementary Table 7).
Effects in the rain. The number of pollen grains remained on anthers (a), the ratio of dead pollen (b), and stigma receptivity (c) of upward- and downward-facing flowers after the exposure to rain. In (c), flowers were hand-pollinated 5 h after the exposure to rain and fruit set was evaluated after ca. 1 month. n = 30 for each day in (a), n = 30 and 15 for D1 and D2, respectively in (b), and n = 12 in (c). Effects in the sun. The number of pollen grains remained on anthers (d), the number of pollen grains that germinated on the stigma (e), and fruit-set rate (f) of upward- and downward-facing flowers after exposure to pollinators. In (e), fruit set was evaluated after ca. 1 month. Values are means ± SE. n = 30 for each day in (d) and (e), and n = 9–14 in (f) (precise n for each time point is listed in Source Data file). In (a–e), asterisks indicate two-sided p values, ***p < 0.001; **p < 0.01; *p < 0.05. Source data are provided as a Source Data file.
We tested whether upward facing increases pollination success under sunny conditions when pollinators are active. The number of pollen grains remained on the anthers of upward-facing flowers was smaller than that of downward-facing flowers (p = 0.0661 and 0.0438 in Sunny D1 and Sunny D2, respectively) (Fig. 4d and Supplementary Table 7), indicating that more pollen grains were removed by pollinators from upward-facing flowers in the sun. Although there was no significant difference in the number of pollen grains germinated on the stigma between upward-facing and downward-facing flowers (p = 0.3972 and 0.7140 in Sunny D1 and Sunny D2, respectively) (Fig. 4e and Supplementary Table 7), the fruit set rate in upward-facing flowers was significantly higher than that in downward-facing flowers (p = 0.0109 in Sunny D1, p < 0.0001 in Sunny D2) (Fig. 4f and Supplementary Table 7), indicating that upward-facing flowers increased pollination success.
Discussion
We revealed that the major underlying mechanisms of weather-driven flower movement involve the differential response of the adaxial and abaxial sides of the flower pedicel caused by weather-dependent tropisms: blue light-triggered positive phototropism and dark/low-light-triggered positive gravitropism in the sun and rain, respectively. For the former, the wavelength of light in which pedicels responded (460 nm) overlaps with that absorbed by phototropin, a key photoreceptor for phototropism33. Positive phototropism has been reported to be common in aboveground shoots4,34,35,36, whereas positive gravitropism is unique to pedicels because aboveground shoots generally exhibit negative gravitropism3,31. The signals were likely to be perceived directly by the pedicels because the pedicels showed tropism even when the flowers were removed (Supplementary Fig. 7). Although the bending of the pedicels occurs during elongation, further studies are required to determine the importance of cell elongation, cell proliferation, and turgor pressure as causes of bending.
Flower pedicels are required to switch the direction of bending within a day because flowers face upward in sunny conditions and downward in rain and at night. The comparison of the two sides of the pedicel suggested that the switching is explained by higher expression levels of the auxin response genes, IAA3 and IAA5, and an auxin efflux carrier, PIN3, on the elongating side during either upward facing by phototropism or downward-facing by gravitropism. The switch between phototropism and gravitropism in controlling flower orientation contrasts with the underlying mechanism of flower heliotropism (e.g. Helianthus annuas and Ranunculus adoneus), in which light-induced tropism and circadian regulation of growth cause changes in flower orientation7,9. Circadian gating of phototropism in the pedicel was observed in A. halleri in this study, presumably increasing reproductive success via higher pollinator visitation to upward-facing flowers by enhancing sensitivity to light during days when pollinator activity was high. Although circadian regulation of flower orientation turned out to require light in this study, the circadian regulation of sunflower florets anthesis has been reported to occur under constant dark conditions37. Our transcriptome data in a natural condition also indicated that the major mechanisms of weather-driven flower movement involve phototropism, gravitropism, auxin response, and cell-wall reconstruction. Because our transcriptome data was collected at a single time point under each weather condition, a time series analysis in a future study will reveal further details, including early acting genes that trigger movement and other genes that are up/downregulated transiently outside of our sampling time range.
We demonstrated that weather-driven flower movement achieves two requirements for reproductive success simultaneously in changing weather conditions: increasing pollinator visitation in sunny conditions and avoiding flower damage in rain. Facing upward increased male and female reproductive success under sunny conditions, suggesting that a larger display of white, clustered, upward-facing flowers attracted more pollinators and promoted a higher level of pollen transfer between plants. Previous studies reported that flower orientation restricts pollinators and constrains pollination efficiency. For example, downward-facing flowers receive reduced pollinator visitation and reproductive success compared to horizontally- or upward-facing flowers because downward-facing flowers are less attractive and less accessible to most pollinators6,17,38,39,40. The accessibility of horizontally and upward-facing flowers may depend on flower shape and pollinator type. Long-tongued pollinators have easier access to horizontally long tubed flowers17,38,39,40, and horizontally oriented zygomorphic flowers increase the legitimate landings of insect pollinators compared to upward-facing flowers6,17,39. The zygomorphic but simple shallow flowers of A. halleri are visited by various functional insect groups, that is, generalised pollination system.
In rainy weather, the downward facing orientation prevented a reduction in male reproductive success, which is consistent with our hypothesis. Although several studies have shown that pollen grains are vulnerable to soaking in distilled water13,14,16,41, our study showed that downward-facing flowers can protect pollen grains from raindrops in the natural population. However, this trend was not detected for female success and stigma receptivity. Instead, wet flowers showed higher female success than dry flowers in rainy weather, which was different from previous research and our hypothesis42. Flowers of A. halleri have a dry stigma covered by a primary cell wall, waxy cuticle, and proteinaceous pellicle43, suggesting that the stigma may be resistant to rain to some extent.
Flower closure is another way to protect flowers from raindrops42,44,45. In the study species, flower closure was not strong enough to protect against raindrops. A possible reason for this weak closure is to avoid the deposition of self-pollen grains. Because A. halleri is self-incompatible, the deposition of self-pollen is likely to interfere with outcrossing and reduce reproductive success in A. halleri. In certain self-compatible species, flower closure promotes delayed self-pollination46,47,48. We cannot answer the question of why A. halleri changes flower orientation according to the weather instead of strongly opening and closing flowers. Further work is needed to determine how weather-dependent flower movement is shared by other plant species and why some species close their flowers while others change their flower direction.
This study found a tight coupling between the underlying mechanism and the adaptive significance of weather-driven flower movement. The mechanism allows flowers to face upward only when three conditions, that is, daytime clock phase, blue light, and warm temperature, are fulfilled, whereas flowers face downward when any one of them is missing. These three conditions coincided with those under which the pollinators became active. This mechanism makes flowers accessible to pollinators when they are available; otherwise, it protects flowers, including during rainfall. The trade-off between pollinator attraction and damage avoidance is thought to create selection pressure and shape the weather-dependent tropism of flower pedicels. The evolution of floral traits has mainly been understood in terms of pollinator attraction; however, recognising a strong trade-off with protection is key to understanding the adaptation of diverse floral traits.
Methods
Plant materials and study site
Arabidopsis halleri subsp. gemmifera is a perennial herb distributed throughout East Asia and Far-East Russia49. Flowers are self-incompatible and pollinated by insects. Small white flowers (6–9 mm in petal length) open in early spring (March–May at the study site), and the flowering season extends for a month. The number of ovules per fruit ranged from 7 to 14. Fruits mature 2 months after flowering.
Field surveys and samplings for transcriptomes were conducted in a natural population of A. halleri at the Monzen, Naka-ku, Taka-cho, Taka-gun, Hyogo Prefecture, Japan (35°05′N, 134°54′E; altitude 140–150 m; Supplementary Fig. 8a)50. Air temperature and light intensity (PPFD; photosynthetic photon flux density) were recorded every minute, with average values recorded every 10 minutes using a quantum sensor (LI190R, LI-COR, Lincoln, NE, USA) and a platinum (Pt) temperature sensor (a PT100 probe in a Ø 3.2 × 100 mm stainless steel tube, Shyowa- sangyo, Japan) on the study site during the study period in 2022 and 2023. Humidity was recorded every minute using a data logger (TM-305U, Fuso, Japan) in 2022. The average high/low temperatures during the study periods were 20.6 °C/7.4 °C and 19.8 °C/7.3 °C for 2022 and 2023, respectively. The average high- and low-humidity levels were 91.9% and 42.2%, respectively, in 2022 (no data are available for 2023).
Field surveys
Flower movements were examined using interval photographs of 7–8 flower stems captured by three time-lapse cameras (H4S Trail Camera, Apeman, Shenzhen, China) set at the study site (Supplementary Fig. 8a). The cameras automatically captured pictures at 10-min intervals. Flower angles with reference to the horizontal axis were measured every 2 h using ImageJ software (http://rsb.info.nih.gov/ij/) to understand flower movement within a day under different weather conditions. Flower angles were measured hourly throughout the day to evaluate the effects of light intensity, temperature, and humidity on flower angles. One to three fresh flowers that opened within a few days from each of the two or three inflorescences per camera were used for measurement. Additionally, we measured the light spectrum (photon flux density at each wavelength) every 10 min during the field survey using a light analyser (LA-105, NK System, Osaka, Japan). As the light intensities measured as PPFD and blue light PFD (400–500 nm) were highly correlated (Supplementary Fig. 8b), we used PPFD for further analyses.
Pedicel curvature (curved angle from the straight pedicel) and pedicel length were measured along flower stages from flower buds (three buds with appearance of petal tips) to withered flowers to evaluate the dependency of pedicel bending on flower stage. We collected 30 flower stems from the study site at 18:30 when fresh flowers faced downward at a level similar to that at night. Each flower with a pedicel was removed from the stem, taped to paper, and photographed using a digital camera (TG-6, Olympus, Tokyo, Japan) for measurement using ImageJ software.
The length of the epidermal cells of the pedicel was measured at three flower stages: before, during, and after pedicel elongation (i.e. flower buds, open flowers, and withered flowers, respectively). The pedicels were preserved in a 1.5 ml tube containing FAA (formaldehyde:ethanol:acetic acid:water = 5:50:5:40 in volume) and stored at room temperature. The pedicels of 11 plants were observed using an optical microscope and three positions of each pedicel were photographed (11 plants × 3 flower stages × 3 positions = 99 photographs). The lengths of five epidermal cells per picture were measured using ImageJ software. The length of the epidermal cells of the pedicel was compared between flowering stages using a generalised linear mixed model (GLMM) with a Gamma error distribution using glmmTMB v.1.1.7 package51. All statistical analyses were performed using R version 4.2.152. The plant ID was set as a random factor in the GLMM. The length of the epidermal cells was the response variable, and the flowering stage (bud, open, or withered) was the explanatory variable. The significance of explanatory variables was evaluated using the Wald chi-square test using the Car package v.3.1-253. Tukey’s test was performed using the multcomp v.1.4-25 package in order to assess the significance level in post-hoc comparisons54.
A single flower from each of the 35, 34 and 34 inflorescences on rainy, cloudy, and sunny days, respectively, was photographed at noon using a digital camera (WG-7, Ricoh, Tokyo, Japan) to quantify flower openness under different weather conditions. We chose typical rainy, cloudy, and sunny days corresponding to continuous precipitation, 80–100% cloud cover without precipitation, and less than 20% cloud cover during the observations, respectively. We quantified flower openness by measuring the angle formed by the open part of the opposite petals using the ImageJ software. The flower openness was compared between weather conditions using a generalised linear model (GLM) with a Gaussian error distribution. The flower openness was the response variable, and weather condition (rainy, cloudy, or sunny) was set as an explanatory variable. The significance levels of the explanatory variables were evaluated using the likelihood ratio test in the Car package. Tukey’s test was performed using the multcomp package to assess the significance level in post-hoc comparisons.
A total of 36 inflorescences each were photographed from a vertical view using a camera (WG-7) at noon on rainy, cloudy, and sunny days to quantify the display size of the flowers under different weather conditions. By placing a black background under the group of flowers, the vertically projected area of the white petals of each inflorescence was measured using ImageJ software. The display size of the flowers was compared between weather conditions using a GLM with a Gaussian error distribution. The vertically projected area of the white petals was the response variable, and weather condition (rainy, cloudy, or sunny) was set as an explanatory variable. The significance levels of the explanatory variables were evaluated using the likelihood ratio test in the Car package. Tukey’s test was performed using the multcomp package to assess the significance level in post-hoc comparisons.
Pollinators were directly observed between 7:30 and 18:05. Two observers walked slowly along two 40 × 5 m belt transects containing flowering plants at the study site. Insects touching anthers and/or pistils were recorded during the 15-min period of each census. In total, 24 and 59 censuses were conducted on the selected 7 days in 2022 and 2023, respectively. The insect groups were recorded in the field. Insects that could not be identified were caught and classified in the laboratory, at least at the order level.
The correlation between the average flower angles and the visiting frequency of pollinators was assessed using Spearman’s rank correlation coefficient. GLMs with Gaussian and negative binomial error distributions were used to evaluate the effects of light intensity (PPFD) and temperature on flower angle and visiting frequency of pollinators, respectively. The flower angle or number of pollinators was a response variable, and the mean light intensity from 2 h before to the measured point, mean temperature from 2 h before to the measured point, and their interactions were set as explanatory variables. The number of observers was set as an offset term in the GLM for the visiting frequency of the pollinators. The best models were selected based on the Akaike information criterion using MASS v.7.3-60 package55. GLM with Gaussian error distributions was used to evaluate the effects of humidity on flower angle. The flower angle was a response variable, and the mean humidity from 2 h before to the measured point was set as an explanatory variable. The significance levels of the explanatory variables were evaluated using the likelihood ratio test in the Car package.
Experiments in the growth chamber
We conducted a series of growth chamber experiments to evaluate the effects of light and circadian rhythms (1), the effect of light colour (2), and temperature (3) on flower movement. Plants used in the three experiments were prepared as follows. Seeds collected from the study site were surface-sterilised and sown on MS agar (FUJIFILM Wako Pure Chemical, Osaka, Japan) in square plastic plates (140 × 100 × 14.5 mm), and stratified in darkness at 4 °C for 7 days in a cold chamber (BMS-501F3, NIHON FREEZER CO., Tokyo, Japan). Plates were then transferred to a growth chamber (Biotron LH-3505-ZZ, NK System) set at 16/8 h light/dark (25°C constant temperature, ~80% of humidity), and 20-day-old seedlings were transplanted into peat pellets (Jiffy-7, 33 mm, Jiffy Products International AS, Stange, Norway) and grown in the same chamber. Then, 40-day-old seedlings were transplanted to soil [a 1:1 mixture of culture soil (Ikubyo-baido; N, P and K = 320, 210 and 300 mg /L, respectively, Takii, Kyoto, Japan) and lightweight shale (Kanuma-soil)] in plastic pots (diameter = 9 cm), and pots were transferred to a growth chamber (CfER-specially-designed, NK System, Osaka, Japan) set at 12/12 h light/dark (22 °C/15 °C) with a light intensity of 240 μmol m–2 s–1 at the pot surface level. The pots were placed in plastic trays with water (1–2 cm depth) for the remainder of the experiment. After growing plants for a week, the plants were vernalized by applying a constant temperature (5 °C), 12/12 h light/dark, and a light intensity of 120 μmol m–2 s–1 for 2 months. After the low-temperature treatment, settings of the growth chamber were reverted to the original settings of 12/12 h light/dark (22 °C/15 °C) with a light intensity of 240 μmol m–2 s–1 to induce bolting. One month later, flowers began to open. Then, flowering plants were transferred to another growth chamber (CfER-specially-designed) with settings of 12/12 h light/dark with a light intensity of 140 μmol m–2 s–1 to evaluate flower movements under the three sets of experimental conditions. The temperature was kept constant at 20 °C, except for the experiments that examined the effects of temperature.
Six plants were placed under the 12/12 h light/dark condition with a light intensity of 140 μmol m–2 s–1 for 3 days and transferred to the continuous light or dark condition for 3 days to evaluate the effects of light and circadian rhythms on the flower movement. Five additional plants were used to evaluate the effect of light intensity during continuous light conditions on the period length of the circadian rhythm and the range and phase of the response. They were placed under the 12/12 h light/dark condition with a light intensity of 140 μmol m–2 s–1 for 3 days and transferred to the continuous high light (410 μmol m–2 s–1) or low light (40 μmol m–2 s–1) for 3 days. During these 6 days, the flower angle was measured every hour by tracking the plants.
Subsequently, 14, 11, 11 and 14 plants were placed under blue (peak/range: 460 nm/420–520 nm), green (516 nm/460–600 nm), yellow (600 nm/550–640 nm) and red (630 nm/580–670 nm) LED lights, respectively, and the flower angle was measured after 4.5 h to evaluate the effect of light colour on flower movement. Ten plants were placed under 10 °C or 20 °C, eight plants were placed under 80% or 99%, and the flower angles were measured 5 h after the light exposure to evaluate the effect of temperature and humidity on the flower movement. The experiments were conducted in 12/12 h light/dark with a light intensity of 140 μmol m–2 s–1.
Flower movements in all the experiments were evaluated as follows. The flower stem of each target plant was clipped onto a vertical stick (diameter = 5.7 mm, length = 40 cm) with sponge cubes (3 cm per side; Supplementary Fig. 8c). Flower movements were recorded using photographs of the inflorescences taken from a horizontal view with two time-lapse cameras (WG-70, Ricoh, Tokyo, Japan). A flashlight covered with green film was used for photography in the dark. Flower angles with reference to the horizontal axis were measured for 1–3 fresh flowers per inflorescence opened within a few days using ImageJ software.
Flower angles were compared between different light colour, temperature, or humidity treatments using GLMMs with a Gaussian error distribution using the glmmTMB package. The flower angle was a response variable, and light colour (blue, green, yellow, or red), temperature (10 °C or 20 °C), or humidity (80% or 99%) was set as an explanatory variable. The plant ID was set as a random factor in the GLMMs. The significance of explanatory variables was evaluated using the Wald chi-square test. Tukey’s test using the multcomp package in R was performed to assess the significance level in post-hoc comparisons.
Transcriptome analyses
Transcriptome analyses were performed on pedicels, leaves, and flowers under rainy and sunny conditions at the study site. Rainy sampling was conducted from 15:00 to 15:30, an hour after the rain began, on 21 April 2022. Sunny sampling was conducted from 14:30 to 15:00 on 10 May 2022. For each rainy and sunny sampling, we chose five plants separated by at least 3 m and collected three to four pedicels of freshly opened flowers, one to two stem leaves, and three to four freshly open flowers from each plant. Samples collected during the 30 min were collectively treated as a single time point. Samples were preserved in the tubes with 700 μL of RNAlater solution (AM7021, Invitrogen, Thermo Fisher Scientific, USA) on ice and transported to the laboratory. After placing at 4 °C overnight, samples were stored at −20 °C for four months until RNA extraction.
The samples were homogenised in lysis/binding buffer using a multibead shocker (Yasui Kikai, Osaka, Japan) for RNA extraction. The mRNA was isolated directly from the homogenate using streptavidin magnetic beads (New England Biolabs, Ipswich, MA, USA, #S1420S) and 5ʹ biotinylated polyT oligonucleotides56. RNA libraries were prepared using the modified Breath Adapter Directional sequencing (BrAD-seq) method57, which was modified to use KAPA HiFi HotStart ReadyMix (Kapa Biosystems, Woburn, MA, USA, #KK2062) in the final PCR. Briefly, the mRNA was heat-fragmented and primed with a 3ʹ adapter-containing oligonucleotide primer targeting the polyA tail of the mRNA. cDNA was synthesised using RevertAid Reverse Transcriptase (Thermo Fisher Scientific, #EP0441) in a ProFlex 3 × 32-well PCR System (Thermo Fisher Scientific). The 5ʹ adapter was added by strand-specific breath capture, and the second strand was synthesised using DNA Polymerase I (Thermo Fisher Scientific, #EP0041). Final PCR enrichment was performed using oligonucleotides containing the full adapter sequence, with a unique index for each sample. PCR products were purified using AMPure XP beads (Beckman Coulter, Brea, CA, USA, #A63881) for size selection. The size distribution and concentration of the library were measured using a 2100 Bioanalyzer (Agilent Technology, Palo Alto, CA, USA) and a Qubit Flex Fluorometer (Thermo Fisher Scientific), respectively. The products from the samples were pooled as libraries and sequenced in two lanes on a HiSeq 2500 instrument (Illumina).
The 50-base single-end reads with index sequences were determined. Pre-processing and quality filtering were performed using fastp v.0.20.058. The pre-processed RNA-seq reads were mapped to the references and quantified using RSEM v.1.2.3159 and Bowtie2 v.2.2.960. The file format of the mapped read files was converted into a compressed binary alignment map format using SAMtools v.1.3.161. The reference sequences used were the nuclear and chloroplast transcript sequences of A. halleri subsp. gemmifera, 8109 viral sequences (NCBI GenBank), and an ERCC spike-in control (Thermo Fisher Scientific). Transcripts of A. halleri (32,553 genes) were annotated using the BLAST best hit against Araport11.
DEGs (fold change >1.2, false discovery rate <0.03) between any of the following two groups were determined using the glmQLFit and glmTreat functions of the edgeR v.3.38.4 package62 in R: (1) between different weather conditions in the same organ (rain vs. sun in pedicel) and (2) between different organs under the same weather conditions (pedicel vs. leaf and pedicel vs. flower in the rain, pedicel vs. leaf, and pedicel vs. flower in the sun). The overlaps between the DEGs were visualised using Venn plots created with the VennDiagram v.1.7.3 package63. The GO annotation of A. thaliana (8 July 2023 version, Gene Ontology Consortium, http://geneontology.org/) was used for the GO enrichment analysis. The annotation of GO identifiers to GO terms was based on the GOTERM dataset of the GO.db v.3.15 package (https://www.bioconductor.org/packages/release/data/annotation/html/GO.db.html). Enrichment was tested using Fisher’s exact test in the R software. The P values were corrected using the Benjamini–Hochberg method and the mt.rawp2adjp function of the multtest v.2.52.0 package. The gene expression patterns of different organs and weather conditions were visualised using a heatmap created with the gplots v.3.1.3 package.
RT-qPCR analyses
The expression of selected genes in the adaxial and abaxial halves of pedicels was compared between those facing upward and downward using RT-qPCR. To make the pedicels face upward and downward, the inflorescences were irradiated with blue light (peak/range, 460 nm/420–520 nm) and set in the dark. In more detail, for facing upwards, flowering plants were exposed to a light intensity of 50 μmol m–2 s–1 (wide-spectrum LEDs for plant growth) from 6:00 a.m. in the growth chamber (CfER-specially-designed, NK System), the blue light was irradiated from 9:00 a.m., and the inflorescence was collected after 30 min. For facing downward, flowering plants were irradiated with blue light from 6:00, the blue light was turned off at 9:00, and the inflorescences were collected in the dark after 30 min. The collected inflorescences were fixed to a glass slide with double-sided tape, and the pedicels were separated into adaxial and abaxial halves using a blade under a stereomicroscope. The adaxial and abaxial halves of pedicels were preserved in the tubes with 700 μL of RNAlater solution. After placing at 4 °C overnight, samples were stored at −20 °C until RNA extraction. Pedicels of 2–6 freshly opened flowers were collected from each of the ten plants for the upward and downward treatments. The plants used here were selected from the ones described in the “Experiments in the growth chamber” section.
Total RNA was extracted automatically using a Maxwell 16 LEV Plant RNA kit (Promega, Madison, WI, USA) and reverse transcribed using a High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). Transcript quantification was performed with Fast SYBR Green Master Mix (Thermo Fisher Scientific) using QuantStudio 7 Flex (Thermo Fisher Scientific). Primers were designed using the sequence of A. halleri as a template using Primer-BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast/; Supplementary Table 8). AhgACTIN2 (AhgACT2) served as the internal control. For the samples, we initially quantified gene expression using three reference genes (AhgACT2, AhgPP2AA3, and AhgUBQ10) and found that the values calculated using AhgACT2 and the geometric mean of the three references were similar (Supplementary Fig. 9). Relative expression levels to the average gene expression of the abaxial part when facing downward were calculated from ΔΔCt values for each target gene64. The relative expression values of the adaxial and abaxial parts were compared using the Wilcoxon signed-rank sum test for each gene and treatment using the exactRankTests v.0.8-35 package (https://cran.r-project.org/web/packages/exactRankTests/index.html) in R.
Application of the inhibitor of PIN function, NPA
Phosphate buffer with the inhibitor (0.1 μM NPA [N-(1-Naphthyl)phthalamic acid, Tokyo Chemical Industry Co., Tokyo, Japan]) or with water (control) was applied to plants in order to evaluate whether flower movement depended on the auxin transportation. The plants were grown using the same procedure described in the ‘Experiments in the growth chamber’ section. A flower stem was collected from each plant, inserted into a 10 mL plastic tube with water, and then secured with a sponge. Cut flowers were sprayed by 0.1 μM NPA or control solution at 10:00 and 16:00 on each day for consecutive four days. Spraying was started at 16:00 on the first day. Flower movements were recorded using photographs of the inflorescences taken from a horizontal view using two time-lapse cameras (WG-70, Ricoh, Tokyo, Japan). A flashlight covered with green film was used for photography in the dark. Flower angles with reference to the horizontal axis at 11:30 and 22:30 were measured for 1–3 fresh flowers per inflorescence opened within a few days using the ImageJ software. We used nine cut flowers from nine plants for each NPA and control treatment.
The flower angles were compared at different times on the third and fourth days within the same treatment using GLMMs with a Gaussian error distribution. The flower angle was the response variable, time and day (10:30 and 22:30 on the third day, and 10:30 and 22:30 on the fourth day) was set as an explanatory variable, and plant ID was set as a random factor. GLMM analysis was performed for each treatment group. The significance of explanatory variables was evaluated using the Wald chi-square test. Tukey’s test was performed in order to assess the significance level of post-hoc comparisons.
Manipulation experiments in the field
We conducted field manipulation experiments to test the adaptive significance of upward- and downward-facing flowers. We used either cut flowers or potted flowering plants; the former was used to evaluate the effects of weather at the flowering stage, and the latter was used when the evaluation was extended to the fruiting stage. For cut flowers, we prepared pairs of upward-facing and downward-facing flowers from individual test plants. Four flower stems per individual were collected from the field before the experiments (evening of the previous day or morning of the experimental day). Two stems were inserted into a 10 mL plastic tube with water and secured with a sponge, resulting in two sets of tubes per plant. Potted plants were grown from seeds collected from the study site and their clonal offspring. The plants were grown using the same procedure described in the ‘Experiments in the growth chamber’ section. After the flowers started to open, the pots were transferred to the outside in the shade for 2 weeks before the experiment to acclimatise them to the outside environment. The plants were placed in boxes covered with a fine-meshed nylon net to exclude insects.
On the morning of the experimental day, we set either the cut flowers or potted plants under high light (irradiated from above with a light intensity of 240 μmol m–2 s–1) and dark (covered with a blackout curtain) conditions for 3 h to prepare the upward- and downward-facing flowers, respectively (Supplementary Fig. 9a). Upward- and downward-facing flowers were placed in either rain or sun to test the effects of weather. We placed tubes with cut flowers and potted flowering plants outside in rain for an hour to expose flowers to raindrops, or in the sun for 2 h to expose flowers to insect pollinators (Supplementary Fig. 9b and c). In the experiments under sunny conditions, we paired upward- and downward-facing flowers separated by 30 cm, and pairs were placed at least 2 m apart at the field site to avoid interference between replicate pairs and to randomise the effects of local insect abundance. Experiments on rainy days (Rainy D1 and Rainy D2) were conducted at approximately noon on 7 and 12 April, respectively, and those on sunny days (Sunny D1 and Sunny D2) were conducted in the afternoon of 9 April and 1 May, respectively, in 2023. Both cut flowers and potted plants were tested on all days except for Rainy D2, when only cut flowers were tested.
Assessment of reproductive success
We performed three measurements (1–3) to assess the effects of flower orientation on flower damage under rainy conditions. (1) Number of remained pollen grains: The pollen grains that remained on the anthers were counted to evaluate the level of protection against pollen lost by rain. On each of Rainy D1 and Rainy D2, 30 pairs of upward- and downward-facing cut flowers were used. Fresh flowers per cut-flower tubes were selected for evaluation. All six anthers of the single flower sample were preserved in a separate 200-μL tube with 70% ethanol 3 h after the exposure to the rain and stored at room temperature until counting. (2) Dead pollen ratio: We evaluated the effect of rain on pollen viability by determining the ratio of dead pollen grains to remained pollen grains in flowers. We selected a fresh flowers per cut-flower tubes, and pollen grains remained were mounted on a slide glass with 1% MTT solution65 ~20 h after the exposure. More than 100 yellow and purple pollen grains per flower were counted using an optical microscope 15 min after the MTT application. We used 30 upward-facing and 30 downward-facing cut flowers on Rainy D1 and 15 upward-facing and 15 downward-facing cut flowers on Rainy D2. The pollen grains were considered viable when they stained purple with MTT (Supplementary Fig. 10a, b). (3) Stigma receptivity: The effect of rain on stigma receptivity was evaluated by measuring fruit set after artificial outcross pollination. Outcross pollination was performed 4 h after rain exposure by depositing pollen grains onto the stigmas of recipient plants using fine forceps. Pollen grains from multiple anthers collected from more than 30 plants at the study site were used. Multiple anthers from different donor individuals were used for a single cross to ensure that the stigma received compatible pollen grains because A. halleri flowers are self-incompatible. The pedicels of the crossed flowers were blue-painted, and fruit set was recorded after ca. one month. The experiment was conducted on Rainy D1 using 12 upward-facing and 18 downward-facing potted plants. We made an artificial crossing on additional flowers as a positive control for fruiting and excluded pots that failed to fruit in the control treatment (one upward-facing and six downward-facing pots).
We made the following three measurements (4–6) to assess the effects of flower orientation on reproductive success under sunny conditions. (4) Number of pollen grains remained: We counted pollen grains that remained on the anthers to evaluate pollen removal by insect pollinators, and fewer remained pollen grains indicated higher male reproductive success. Fresh flowers per cut flower tube were selected for evaluation. All six anthers of the single flower sample were preserved in a separate 200-μL tube with 70% ethanol after the exposure to the sun and stored at room temperature until counting. We used 29 pairs of upward-facing and downward-facing cut flowers from Sunny D1 and 30 pairs from Sunny D2. (5) The number of pollen grains germinated on the stigmas and the number of compatible pollen grains deposited on the stigma during exposure to insect pollinators were evaluated. After treatment, the cut flowers were placed in a box covered with a net for 24 h to avoid further insect visits and to allow compatible pollen grains to germinate. Fresh flowers per cut flower tube were selected for evaluation. A pistil of the single flower sample was preserved in a separate 200-μL tube containing FAA and stored at room temperature until counting. We used 29 upward-facing and 28 downward-facing cut flowers from Sunny D1 and 30 pairs from Sunny D2. (6) Fruit set rate: We evaluated the female reproductive success of flowers based on the fruit set after exposure to insect pollinators. The pedicels of the target flowers of the potted plants were red-painted, and the fruit set was recorded after ca. one month. We used 16 pairs of upward-facing and downward-facing potted plants from Sunny D1 and 11 pairs from Sunny D2. We made artificial crossings on additional flowers as a positive control of fruiting and excluded pots that failed fruiting in the control treatment (two upward-facing and six downward-facing pots on Sunny D1 and one upward-facing and two downward-facing pots on Sunny D2).
Samples in a 200 μL tube containing 70% ethanol were sonicated for 45 min to release pollen grains from anthers to count the pollen grains that remained on the anthers in (1) and (4). The mixture was transferred to a glass-counting chamber filled with 50 mL of an electrolyte solution (Coulter Isoton II diluent; Beckman Coulter, CA, USA), and pollen grains were counted using a particle counter (Z2 Coulter Particle and Size Analyser; Beckman Coulter). The average values of the five assays for each sample were calculated. Pistil samples were softened in 8 M NaOH for 20 min at 65 °C, rinsed with distilled water, and stained for 20 min in aniline blue (0.1%, dissolved in 0.1 M K3PO4) at 65°C to count the pollen grains germinated on stigma in (5). Each stained pistil was placed on a glass slide with a drop of glycerol, squashed carefully with a coverslip, and observed under a fluorescence microscope (BX43; Olympus, Tokyo, Japan) using filter cubes (340–390 nm excitation and 420 nm emission; U-FUW; Olympus). Pollen grains germinated on the stigma were counted (Supplementary Fig. 10c).
The above indicators (1–6) of flower damage and reproductive success were compared between flower orientations for each experimental day using GLMs and GLMMs. For the number of pollen grains remained on the anthers (1 and 4) and germinated on the stigma (5), GLMMs postulating a negative binomial error distribution with a log-link function were performed. For dead pollen ratio (2), GLMMs postulating a binomial error distribution with a logit-link function were performed. For stigma receptivity (3), GLMs, postulating a binomial error distribution with a logit link function, were performed. For the fruit set rate (6), GLMMs postulating a binomial error distribution with a logit-link function were performed. In each model, each indicator variable was a response variable and flower orientation (upward or downward-facing) was set as an explanatory variable. Plant ID was set as a random factor in the GLMMs in (1), (2), (4) and (5). The pair ID is set as a random factor in the GLMM in (6). The significance levels of the explanatory variables were evaluated using the likelihood-ratio test and Wald chi-square test using the Car package in the GLM and GLMM, respectively.
Statistics and reproducibility
Procedures for statistical analysis and sample size selection were explained in the individual methods sections. Data exclusion was explained in the section ‘Assessment of reproductive success’. The growth chamber and field experiments were randomised, but the field observations were not randomised between different weather conditions because we used open flowers for each day. Investigators were not blinded to allocation during the study.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The RNA-seq data generated in this study have been deposited in the DNA Data Bank of the Japan (DDBJ) BioProject database under accession code PRJDB16819. The source data generated in this study are provided in Source Data 1-5. Raw data of field surveys, laboratory experiments, gene expressions (RT-qPCR and RNA-seq) are deposited in figshare repository [https://doi.org/10.6084/m9.figshare.28162829]. Source data are provided with this paper.
Code availability
The R code used in this study was deposited in figshare repository [https://doi.org/10.6084/m9.figshare.28162829].
References
Žádníkova, P., Smet, D., Zhu, Q., Van Der Straeten, D. & Benková, E. Strategies of seedlings to overcome their sessile nature: auxin in mobility control. Front. Plant Sci. 6, 218 (2015).
Whippo, C. W. & Hangarter, R. P. Phototropism: bending towards enlightenment. Plant Cell 18, 1110–1119 (2006).
Fukaki, H., Fujisawa, H. & Tasaka, M. Gravitropic response of inflorescence stems in Arabidopsis thaliana. Plant Physiol. 110, 933–943 (1996).
Liscum, E. et al. Phototropism: growing towards an understanding of plant movement. Plant Cell 26, 38–55 (2014).
Armbruster, W. S. & Muchhala, N. Floral reorientation: the restoration of pollination accuracy after accidents. N. Phytol. 227, 232–243 (2020).
Ushimaru, A. & Hyodo, F. Why do bilateral symmetrical flowers orient vertically? Flower orientation influences pollinator landing behavior. Evol. Ecol. Res. 7, 151–160 (2005).
Stanton, M. L. & Galen, C. Blue light controls solar tracking by flowers of an alpine plant. Plant Cell Environ. 16, 983–989 (1993).
Serrano, A. M. et al. Following the star: Inflorescence heliotropism. Environ. Exp. Bot. 147, 75–85 (2018).
Atamian, H. S. et al. Circadian regulation of sunflower heliotropism, floral orientation, and pollinator visits. Science 353, 587–590 (2016).
Brooks, C. J., Atamian, H. S. & Harmer, S. L. Multiple light signaling pathways control solar tracking in sunflowers. PLoS Biol. 21, e3002344 (2023).
Stanton, M. L. & Galen, C. Consequences of flower heliotropism for reproduction in an alpine buttercup (Ranunculus adoneus). Oecologia 78, 477–485 (1989).
Kudo, G. Ecological significance of flower heliotropism in the spring ephemeral Adonis ramosa (Ranunculaceae). Oikos 72, 14–20 (1995).
Mao, Y. Y. & Huang, S. Q. Pollen resistance to water in 80 angiosperm species: flower structures protect rain-susceptible pollen. N. Phytol. 183, 892–899 (2009).
Wang, Y., Meng, L. L., Yang, Y. P. & Duan, Y. W. Change in floral orientation in Anisodus luridus (Solanaceae) protects pollen grains and facilitates development of fertilized ovules. Am. J. Bot. 97, 1618–1624 (2010).
Haverkamp, A. et al. Flower movement balances pollinator needs and pollen protection. Ecology 100, 1–11 (2019).
Nakata, T., Rin, I., Yaida, Y. A. & Ushimaru, A. Horizontal orientation facilitates pollen transfer and rain damage avoidance in actinomorphic flowers of Platycodon grandiflorus. Plant Biol. 24, 798–805 (2022).
Yu, Y., Li, X., Xie, D. & Wang, H. Horizontal orientation of zygomorphic flowers: significance for rain protection and pollen transfer. Plant Biol. 23, 156–161 (2021).
Lawson, D. A. & Rands, S. A. The effects of rainfall on plant–pollinator interactions. Arthropod. Plant Interact. 13, 561–569 (2019).
Vicens, N. & Bosch, J. Weather-dependent pollinator activity in an apple orchard, with special reference to Osmia cornuta and Apis mellifera (Hymenoptera: Megachilidae and Apidae). Environ. Entomol. 29, 413–420 (2000).
Karbassioon, A. et al. Responses in honeybee and bumblebee activity to changes in weather conditions. Oecologia 201, 689–701 (2023).
Hashiguchi, Y. et al. A unique HEAT repeat-containing protein SHOOT GRAVITROPISM6 is involved in vacuolar membrane dynamics in gravity-sensing cells of Arabidopsis inflorescence stem. Plant Cell Physiol. 55, 811–822 (2014).
Morita, M. T., Saito, C., Nakano, A. & Tasaka, M. Endodermal-amyloplast less 1 is a novel allele of SHORT-ROOT. Adv. Space Res. 39, 1127–1133 (2007).
Nakamura, M., Toyota, M., Tasaka, M. & Morita, M. T. An Arabidopsis E3 ligase, SHOOT GRAVITROPISM9, modulates the interaction between statoliths and f-actin in. Plant Cell 23, 1830–1848 (2011).
Strohm, A. K., Baldwin, K. L. & Masson, P. H. Multiple roles for membrane-associated protein trafficking and signaling in gravitropism. Front. Plant Sci. 3, 1–12 (2012).
Taniguchi, M. et al. The arabidopsis LAZY1 family plays a key role in gravity signaling within statocytes and in branch angle control of roots and shoots. Plant Cell 29, 1984–1999 (2017).
Stortenbeker, N. & Bemer, M. The SAUR gene family: the plant’s toolbox for adaptation of growth and development. J. Exp. Bot. 70, 17–27 (2019).
Wang, X. et al. The asymmetric expression of SAUR genes mediated by ARF7/19 promotes the gravitropism and phototropism of plant hypocotyls. Cell Rep. 31, 107529 (2020).
Taniguchi, M., Nakamura, M., Tasaka, M. & Morita, M. T. Identification of gravitropic response indicator genes in Arabidopsis inflorescence stems. Plant Signal Behav. 9, 6–11 (2014).
Boron, A. K. et al. Over-expression of AtEXLA2 alters etiolated arabidopsis hypocotyl growth. Ann. Bot. 115, 67–80 (2015).
Ding, Z. et al. Light-mediated polarization of the PIN3 auxin transporter for the phototropic response in Arabidopsis. Nat. Cell Biol. 13, 447–452 (2011).
Rakusová, H. et al. Polarization of PIN3-dependent auxin transport for hypocotyl gravitropic response in Arabidopsis thaliana. Plant J. 67, 817–826 (2011).
Abas, L. et al. Naphthylphthalamic acid associates with and inhibits PIN auxin transporters. Proc. Natl. Acad. Sci. USA 118, 1–8 (2020).
Christie, J. M., Blackwood, L., Petersen, J. & Sullivan, S. Plant flavoprotein photoreceptors. Plant Cell Physiol. 56, 401–413 (2015).
Fankhauser, C. & Christie, J. M. Plant phototropic growth. Curr. Biol. 25, R384–R389 (2015).
Haga, K. & Sakai, T. PIN auxin efflux carriers are necessary for pulse-induced but not continuous light-induced phototropism in Arabidopsis. Plant Physiol. 160, 763–776 (2012).
Kong, Y. & Zheng, Y. Phototropin is partly involved in blue-light-mediated stem elongation, flower initiation, and leaf expansion: a comparison of phenotypic responses between wild Arabidopsis and its phototropin mutants. Environ. Exp. Bot. 171, 103967 (2020).
Marshall, C. M., Thompson, V. L., Creux, N. M. & Harmer, S. L. The circadian clock controls temporal and spatial patterns of floral development in sunflower. eLife 12, e80984 (2023).
Fenster, C. B., Armbruster, W. S. & Dudash, M. R. Specialization of flowers: Is floral orientation an overlooked first step? N. Phytol. 183, 502–506 (2009).
Wang, H. et al. Change of floral orientation affects pollinator diversity and their relative importance in an alpine plant with generalized pollination system, Geranium refractum (Geraniaceae). Plant Ecol. 215, 1211–1219 (2014).
Yon, F., Kessler, D., Joo, Y. & Cort, L. Fitness consequences of altering floral circadian oscillations for Nicotiana attenuata. J. Integr. Plant Biol. 59, 180–189 (2017).
Huang, S.-Q., Takahashi, Y. & Dafni, A. Why does the flower stalk of Pulsatilla cernua (Ranunculaceae) bend during anthesis? Am. J. Bot. 89, 1599–1603 (2002).
Abdusalam, A. & Tan, D. Y. Contribution of temporal floral closure to reproductive success of the spring-flowering Tulipa iliensis. J. Syst. Evol. 52, 186–194 (2013).
Edlund, A. F., Swanson, R. & Preuss, D. Pollen and stigma structure and function: the role of diversity in pollination. Plant Cell 16, 84–97 (2004).
Bynum, M. R. & Smith, W. K. Floral movements in response to thunderstorms improve reproductive effort in the alpine species Gentiana algida (Gentianaceae). Am. J. Bot. 88, 1088–1095 (2001).
Hase, A., Von, Cowling, R. M. & Ellis, A. G. Petal movement in cape wildflowers protects pollen from exposure to moisture. Plant Ecol. 184, 75–87 (2006).
Dart, S. & Eckert, C. G. Experimental manipulation of flowers to determine the functional modes and fitness consequences of self-fertilization: unexpected outcome reveals key assumptions. Funct. Ecol. 27, 362–373 (2013).
Domingos-Melo, A., Nadia, T., de, L. & Machado, I. C. At the beginning and at the end: combined mechanisms of prior and delayed self-pollination interact to make a “winner” species. Flora.: Morphol., Distrib., Funct. Ecol. Plants 249, 24–30 (2018).
Goodwillie, C. & Weber, J. J. The best of both worlds? A review of delayed selfing in flowering plants. Am. J. Bot. 105, 641–655 (2018).
Honjo, M. N. & Kudoh, H. Arabidopsis halleri: a perennial model system for studying population differentiation and local adaptation. AoB PLANTS 11, plz076 (2019).
Kudoh, H., Honjo, M. N., Nishio, H. & Sugisaka, J. The Long-Term "In Natura" Study Sites of Arabidopsis halleri for Plant Transcription and Epigenetic Modification Analyses in Natural Environments. in Methods Mol Biol. 1830, Plant Transcription Factors. (ed. Yamaguchi, N.) 41–57 (Humana, New York, 2018)
Brooks, M. E. et al. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R. J. 9, 378–400 (2017).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, 2022).
Fox, J. & Weisberg, S. An R Companion to Applied Regression (Sage, Thousand Oaks, 2019).
Hothorn, T., Bretz, F. & Westfall, P. Simultaneous inference in general parametric models. Biom. J. 50, 346–363 (2008).
Venables, W. & Ripley, B. Modern Applied Statistics with S (Springer, New York, 2002).
Kumar, R. et al. A high-throughput method for Illumina RNA-Seq library preparation. Front. Plant Sci. 3, 1–10 (2012).
Townsley, B. T., Covington, M. F., Ichihashi, Y., Zumstein, K. & Sinha, N. R. BrAD-seq: Breath Adapter Directional sequencing: a streamlined, ultra-simple and fast library preparation protocol for strand specific mRNA library construction. Front. Plant Sci. 6, 1–11 (2015).
Chen, S., Zhou, Y., Chen, Y. & Gu, J. Fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884–i890 (2018).
Li, B. & Colin, D. N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinforma. 12, 323 (2011).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Li, H. et al. The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2009).
Chen, H. & Boutros, C. P. VennDiagram: a package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinforma. 12, 35 (2011).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25, 402–408 (2001).
Rodriguez-riano, T. & Dafni, A. New procedure assess pollen viability. Sex Plant Reprod. 241–244 (2000).
Acknowledgements
We thank Dr. T. Muranaka for discussions in the early stages of the study and M. Mihara and T. Horiuchi for their assistance in sequence data preparation. This study was supported by a JSPS Grant-in-Aid for Specially Promoted Research JP21H04977 (H.K. and M.N.H.), JST CREST JPMJCR15O1 (H.K.), and a Nakatsuji Foresight Foundation Research Grant (A.S.).
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Xiaodong Xu and the other, anonymous, reviewer for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shibata, A., Yumoto, G., Shimizu, H. et al. Flower movement induced by weather-dependent tropism satisfies attraction and protection. Nat Commun 16, 4132 (2025). https://doi.org/10.1038/s41467-025-59337-6
Received: 03 June 2024
Accepted: 18 April 2025
Published: 03 May 2025
DOI: https://doi.org/10.1038/s41467-025-59337-6
.png)