Introduction
Humans are a remarkably cooperative species, with unrelated individuals and groups readily able to establish and maintain complex systems of mutual benefit1,2,3. The uniqueness and success of human cooperation has led to a host of theoretical and empirical work into the cognitive and emotional mechanisms required to support it4,5,6; candidate mechanisms include empathic concern and other prosocial motivations7,8,9, an ability to distinguish cooperative versus non-cooperative actions and individuals10, a preference for prosocial acts and actors and/or aversion to antisocial ones11, and the acceptance of and/or desire for the punishment of the antisocial12,13. Given the key role of each of these phenomena within human moral systems across cultures, this work has led to the broader and more substantial claim that human morality stems, at least in part, from evolved adaptations for sustaining cooperation within large groups14,15,16,17,18,19.
Potentially consistent with these claims, developmental psychologists have recently documented various socio-morally relevant phenomena in young infants who may be too young to have acquired such tendencies via learning alone20. For instance, infants as young as 3 months show concern for others in distress that predicts subsequent prosocial behaviours21, and from 4 months show baseline expectations for fairness between novel social agents22,23,24. Infants have also been shown to preferentially attend to and approach a variety of more ‘prosocial’ over more ‘antisocial’ agents, including those who align their behaviours with those of other agents25,26,27 (from 4.5 months), help versus hinder others’ goals (from 3 months)28,29,30, distribute resources fairly over unfairly (from 4 months)21,22,23,24,25,26,27,28,29,30,31,32,33, affiliate with versus aggress against others (from 10 months)34,35, and protect victims versus fail to do so (from 6 months)36. Within these studies, non-social control versions of the same events have not led to selectivity, suggestive that infants’ responses are based on relative prosociality as opposed to lower-level aspects of the stimuli.
The evidence for infants’ selective responses to the sociomoral world amassed to date is broadly consistent with claims for the evolution of human morality14,15,16,17,18,19, and has led some to argue that it reflects an “innate moral core”20 in which some aspects of human morality emerge independently of experience with morally relevant input. That said, current evidence nevertheless falls short of providing irrefutable evidence of such claims in at least two ways. First, as several scholars have noted25, preverbal infants’ responses to morally relevant acts could stem from relatively simpler mechanisms, ones that are social but not moral in nature26,27. For instance, perhaps infants who prefer prosocial others are identifying those who align themselves with other social agents (by matching their actions or adopting their goals)26,27,28, rather than engaging in any higher-level moral assessments of the goodness or rightness of those acts. Although there are a few studies to date that have attempted to address this possibility by pitting equally social, but more- versus less-moral, characters against each other via manipulations of prosocial and antisocial others’ intentions29,30,31,32,33,34,35,36,37,38, and have found evidence for moral responding, those studies were necessarily complex and have typically examined infants from late in the first year and beyond for whom considerable learning and development has undoubtedly occurred. Thus, whether or not infants’ earliest responses are truly “moral” remains very much an open question.
Irrespective of the social versus moral nature of infants’ responses, a second reason that infants’ sociomoral responses fall short of providing evidence for an innate moral core is that even the very youngest infants tested in the studies reviewed above all have at least 3 months of experience in the postnatal environment, and in many cases much more, rendering the relative roles of innate and learned abilities unclear9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42. To date, evidence with human newborns, who possess extremely limited experience with sociomoral phenomena relative to older infants, suggests that selective responses to some aspects of the social world may emerge independently from experience. For instance, newborns become distressed when listening to another newborn’s cry relative to their own cry or matched control sounds43,44,45, argued to be a form of primitive empathy. Newborns also show spontaneous preferences for social stimuli over non- or less-social comparisons; looking longer at faces over scrambled faces46,47,48,49, direct over indirect gaze50, and biological over scrambled motion51. Newborns have also been shown to prefer specific dynamic cues relevant to animacy perception52,53,54, as well as physically possible, goal-directed manual actions over impossible and/or non-goal directed ones55,56,57,58.
The social responsivity and selectiveness in human newborns described above falls well short of evidence for innate morality. Nevertheless, more recent evidence on newborns’ responsiveness to cues that distinguish different types of social interaction gets somewhat closer. For instance, a very recent study found that newborn humans looked more at point-light-walker displays of adult humans that appeared to walk toward them (e.g., approach) rather than away from them (e.g., avoid), and more at point-light walker approach (e.g., social approach) than at approach performed by scrambled point-light displays (e.g., non-social approach). These results were argued to indicate a predisposition for cues indicating social closeness or against cues indicating social avoidance, at least when those cues are directed at newborns themselves59. Further, similarly to a host of recent findings demonstrating that older human infants and adults show dedicated processing of face-to-face versus back-to-back social configurations60,61,62, female visually naive newborn chicks (that is, chicks deprived of all relevant visual input) preferentially approach face-to-face over back-to-back (chicken) biological motion63, suggestive that orientation to meaningful social interactions can arise independently from relevant input at least in a bird model. Finally, and most relevant to the biological roots of sociomoral evaluation, female visually naïve newborn chicks may avoid an aggressive agent relative to its victim64, suggestive of unlearned abilities to distinguish different types of social interactions and/or social roles.
In sum, findings with relatively inexperienced vertebrates (infant humans, newborn humans, and female newborn chicks) suggest that at least some of the abilities required for understanding and evaluating morally relevant stimuli may be unlearned, emerging in the absence of extensive sociomoral experiences. That said, the vast majority of this evidence comes from older human infants, whose sociomoral experiences are obviously limited but far from nonexistent. To our knowledge, no previous study of human newborns has demonstrated sensitivity to behaviours with any sociomoral relevance at all.
In the current studies, including a preregistered replication, we find that 5-day-old newborns attend longer toward prosocial than antisocial interactions looping continuously on either side of a display. Newborns attend more to prosocial interactions both for extremely simple, clearly non-moral forms of prosocial versus antisocial acts (approach versus avoidance; Experiment 1); and for interactions that are more complex and that possess some moral value (helping versus hindering; Experiments 2; and a partial replication study of Experiment 2, Experiment 3). Crucially, 5-day-olds watching matched pairs of non-social control versions of the same displays show no significant preference, suggesting that newborns’ responses in the social conditions are not the result of low-level features of the displays. By just days after birth, then, humans are already sensitive to a crucial distinction within the sociomoral world: the difference between prosocial and antisocial acts.
Results
Preliminary validation experiment
To validate the simplified social stimuli created for newborns, we assessed adults’ explicit interpretations of the prosocial/antisocial and matched non-social control events by showing students two minutes of each side-by-side set of videos shown to newborns (see detailed descriptions below and in Supplementary Information). Each adult was randomly assigned to watch videos from both social conditions (e.g., approach/avoid and help/hinder) or videos from both non-social control conditions in a mixed design. After watching the videos, participants were asked to write down “what happened” during the event on each side of the screen, as well as what “kind of thing” was each circle within each event. As predicted, students almost exclusively (>95% of descriptions) described the social videos in social terms, referencing approach/avoidance or helping/hindering appropriately. In contrast, very few adults (10 %) described the non-social videos in social terms, instead referencing distinct patterns of motion. Descriptions of the characters in the social condition included frequent use of both social and moral terms (e.g., friendly, helpful, altruistic, good, unfriendly, aggressive, bad) whereas descriptions in the non-social condition essentially never used these terms (for a table of all adult descriptions see Source Data file). These descriptions suggest that our stimuli, though highly simplified, are nevertheless interpretable as instances of social and/or moral interactions to adult participants.
Experiment 1
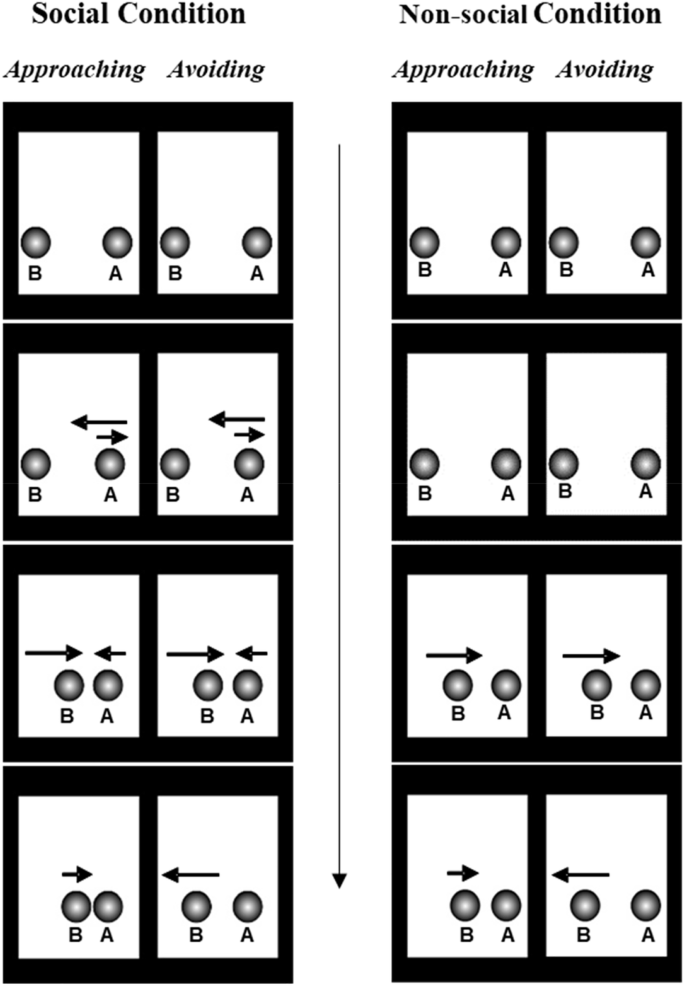
Experiment 1 examined newborns’ sensitivity to an extremely basic cue to relative prosociality: whether an agent tends to approach or avoid another agent. Specifically, newborns watched two 60 s trials involving side-by-side videos each depicting two faceless grey balls; the movements of the balls depicted approach on one side and avoidance on the other. In the social condition, both balls displayed self-propelled motion and appeared agentic. In the social approach video Agent B moved toward Agent A twice, ending in close proximity to it; in the social avoidance video Agent B moved toward and then away from Agent A, ending farther away from it. In the non-social condition, only one of the balls (Agent B) displayed self-propelled motion and appeared agentic; it acted identically to Agent B in the social condition while the other ball remained inert. Thus, the videos depicted approach and avoidance, but of an inert object (see Fig. 1).
The first and last frames depict the start and ending positions of each ball, respectively. In the Social condition, agent A (the ‘recipient’) moves first, while agent B (the ‘approacher’/’avoider’) moves second. Agent B approaches agent A in the approaching event, and avoids agent A in the avoiding event. In the Non-social condition, movements are similar to those performed by agents in the Social condition, except that agent A is an inert object so only agent B moves.
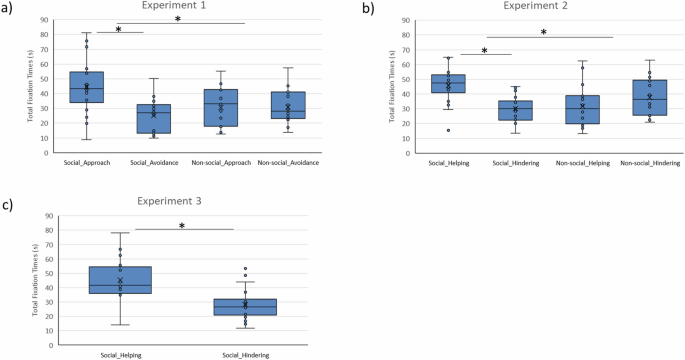
Newborns’ attention toward each side of the display was coded offline by a coder blind to condition. For each trial, coding continued for the entire 60-second trial or until the infant looked away for 10 consecutive seconds, suggesting they lost interest. A repeated measure analysis of variance (rmANOVA) of the total fixation times with Trial (first vs. second) and Action Type (approach vs. avoidance) as within-subject factors, and Condition (social vs. non-social) as a between-subjects factor revealed a significant main effect of Trial (F (1,34) = 6.483, p = 0.016, ηp2 = 0.160), and of Action Type (F (1,34) = 8.071, p = 0.008, ηp2 = 0.192). The interaction Action Type X Condition was also significant (F (1,34) = 8.378, p = 0.007, ηp2 = 0.198); no other statistically significant main effects or interactions were observed: Trial X Action Type F (1,34) = 0.209, p = 0.963, ηp2 = 0.001, Trial X Condition F (1,34) = 0.903, p = 0.763, ηp2 = 0.003, and Trial X Action Type X Condition F (1,34) = 0.209, p = 0.651, ηp2 = 0.006. Follow-up t-tests indicated that newborns looked longer during the first trial (M1 = 35.1 s; SD1 = 12.1) versus the second trial (M2 = 30.4 s; SD2 = 10.1; t (1,35) = 2.580, p = 0.014, d = 0.428, 95% CI [1.008, 8.462]), as well as longer toward approach (Mapproach = 37.6 s; SDapproach = 17.4) versus avoidance (Mavoidance = 27.9 s; SDavoidance = 11.9 actions; t (1,35) = 2.582, p = 0.014, d = 0.660, 95% CI [2.063, 17.248]). Crucially, planned contrasts exploring the significant interaction between Action Type and Condition revealed that in the social condition newborns looked longer at approach (Msocial_approach = 44.5 s; SDsocial_approach = 18.8) than avoidance (Msocial_avoidance = 25.1 s; SDsocial_avoidance = 11.6; t (1,17) = 3.624, p = 0.002, d = 0.883, 95% CI [8.146, 30.842]); there was no statistically significant difference between newborns’ total fixation times toward approach and avoidance in the non-social condition (Mnon-social_approach = 30.6 s; SDnon-social_approach = 13.1; Mnon-social_avoidance = 30.8 s; SDnon-social_avoidance = 11.3; t (1,17) = −0.044, p = 0.966, d = −0.017, 95% CI [−8.952, 8.588]) (Fig. 2a). These effects also emerged at the level of individual infants: 14 of the 18 newborns (77.7%; p = 0.030, binomial test, two-tailed) in the social condition looked longer at approach, whereas only 8 of the 18 newborns (44.4%; p = .814, binomial test, two-tailed) in the non-social condition did so. The difference in rate of preferring approach across conditions was significant, X2(1, N = 36) = 4.208, p = 0.040.
a Experiment 1: total looking time toward the approach event (first box) and the avoidance event (second box) in the social condition (first set of boxes) and the non-social condition (second set of boxes; (N = 18 per set). b Experiment 2: total looking time toward the helping event (first box) and the hindering event (second box) in the social condition (first set of boxes) and the non-social condition (second set of boxes; (N = 18 per set). c Experiment 3: total looking time toward the helping event (first box) and the hindering event (second box) in the social condition (N = 18). Box plots show the median centre line and 25/75 percentiles. Whiskers show min and max values. Internal data points that fall within the whiskers (between the lower quartile −1.5IQR and the upper quartile +1.5IQR) are considered “within the range” of the data. External data points that fall outside the whiskers are considered outliers. Asterisks indicate significant differences of the infants’ total looking times observed with paired t-tests, two-tailed (*p < 0.05).
Results from Experiment 1 provide evidence that human newborns distinguish between an extremely simple form of prosocial versus antisocial interaction, and selectively attend to the prosocial one. That said, this result is limited to approach/avoidance behaviours, which may signal an agent’s baseline orientation toward other agents, but do not themselves possess moral content.
Experiment 2
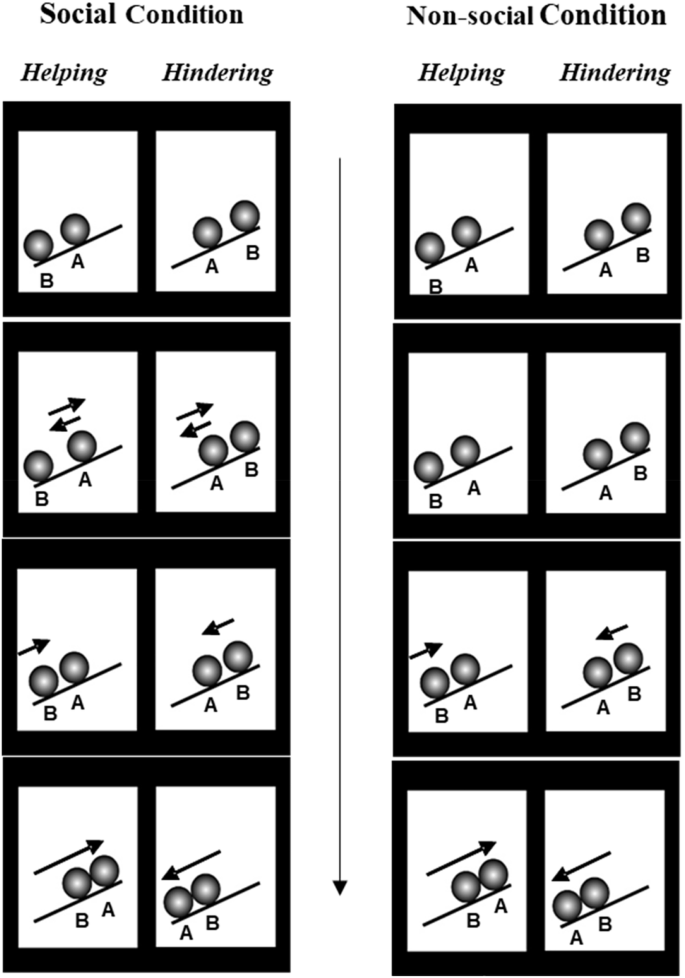
Experiment 2 explored what is arguably a more morally relevant cue to relative prosociality, one agent helping or hindering another, utilizing a highly simplified version of a common display from the literature29,30,31,65. At the start of the social helping event, agent A rests in the middle of an inclined plane and agent B at the bottom. Agent A makes two small movements toward the top of the plane as though trying to move up, and then moves back to its original position each time as though falling backward. Agent B then moves up the plane, comes into contact with agent A, and pushes it to the top as though helping. At the start of the social hindering event, agent A rests in the middle of the plane and agent B at the top. Agent A moves up and falls down the plane twice, and then agent B moves down toward agent A and pushes it to the bottom as though hindering. In the non-social condition, all approach/pushing movements by agent B were identical to those in the social condition. However, because the other ball remained inert, the movements depicted only upward/downward pushing of an object as opposed to helping/hindering of an agent (see Fig. 3).
The first and last frames depict the start and ending positions of each ball, respectively. In the Social condition, agent A (the ‘climber’) moves first, while agent B (the ‘helper’/’hinderer’) moves second. Agent B helps agent A in the helping event and hinders agent A in the hindering event. Movements in the Non-social condition are very similar, except that agent A is an inert object so only agent B moves.
A rmANOVA of total fixation times was performed with Trial (first vs. second) and Action Type (helping vs. hindering) as within-subject factors, and Condition (social vs. non-social) as a between-subjects factor. There were no statistically significant main effects of Trial (F (1,34) = 3.186, p = 0.083, ηp2 = 0.086), Action Type (F (1,34) = 2.088, p = 0.158, ηp2 = 0.058) or Condition (F (1,34) = 2.213, p = 0.146, ηp2 = 0.061); the interactions Action Type X Condition (F (1,34) = 10.365, p = 0.003, ηp2 = 0.234), and Trial X Action Type X Condition (F (1,34) = 8.511, p = 0.006, ηp2 = 0.200) were significant. No other statistically significant interactions were observed, Trial X Condition F (1,34) = 0.436, p = 0.514, ηp2 = 0.013, and Trial X Action Type F (1,34) = 0.001, p = 0.974, ηp2 = 0.001 (Fig. 2b). Planned t-tests indicated that newborns in the social condition looked longer at helping (Msocial_helping = 45.6 s; SDsocial_helping = 11.9) than at hindering (Msocial_hindering = 30.1 s; SDsocial_hindering = 8.7; t (1,17) = 3.730, p = 0.002, d = 1.53, 95% CI [6.760, 24.361]), but that there was no statistically significant difference in newborns’ attention to ‘helping’ (Mnon-social_helping = 31.6 s; SDnon-social_helping = 13.8) versus ‘hindering’ (Mnon-social_hindering = 37.5 s; SDnon-social_hindering = 12.7) in the non-social condition t (1,17) = −1.137, p = 0.271, d = −0.45, 95% CI [−16.907, 5.067]). These effects also emerged at the level of individual infants: 14 of the 18 newborns in the social condition looked longer at helping (77.7%; p = 0.030, binomial test, two-tailed), whereas only 6 of the 18 in the non-social condition did so (33.3 %; p = 0.237, binomial test, two-tailed); this difference was significant, X2(1, N = 36) = 7.200, p = 0.007.
Planned t-tests exploring the significant (though unexpected) interaction Trial X Action Type X Condition revealed that in the social condition newborns looked longer at helping (M1social_helping = 27.5 s; SD1social_helping = 13.6) than hindering in the first test trial (M1social_hindering = 12.8 s; SD1social_hindering = 9.6; t (1,17) = 2.819, p = 0.012, d = 5.17, 95% CI [3.687, 25.626]), while there was no significant difference in attention in the second trial (M2social_helping = 18.1 s; SD2social_helping = 8.4; M2social_hindering = 17.2 s; SD2social_hindering = 8.3; t (1,17) = 0.251, p = 0.650, d = 0.44, 95% CI [−6.688, 8.497]). In the non-social condition, newborns showed an opposite pattern of results, looking longer at ‘hindering’ (M1non-social_hindering = 22.8 s; SD1non-social hindering = 10.9) than at ‘helping’ in the first trial (M1non-social helping = 12.9 s; SD1non-social helping = 7.02; t (1,17) = −2.622, p = 0.018, d = 4.51, 95% CI [−18.029, 1.952]), and showing no significant statistically difference in the second trial (M2non-social_helping = 18.7 s; SD2non-social_helping = 8.6; M2non-social_hindering = 14.6 s; SD2non-social_hindering = 10.4; t (1,17) = 1.120, p = 0.278, d = 1.76, 95% CI [−3.597, 11.739]).
Experiment 3
Experiment 3 was a preregistered replication of the social helping/hindering condition, thereby partially replicating Experiment 2 (https://osf.io/p84br). All methods and relevant analyses were identical. Although we originally pre-registered also replicating the non-social condition, we noted that that might not be possible given the first author was moving universities and would soon lose access to testing newborns. We stated that we would therefore run the social condition first, because it presented the main effect of interest. We were only able to run the social condition prior to A.G.’s move; at the time of final submission we were still unable to access newborns.
A rmANOVA of the total fixation times with Trial (first vs. second) and Action Type (helping vs. hindering) as within-subject factors showed a significant main effect of Action Type (F (1,17) = 11.956, p = 0.003, ηp2 = 0.413), but no statistically significant main effect of Trial (F (1,17) = 0.197, p = 0.663, ηp2 = 0.011) nor interaction between Trial and Action Type (F (1,17) = 0.008, p = 0.932, ηp2 = 0.001) was observed (Fig. 2c). Replicating Experiment 2, a planned contrast revealed that newborns looked longer at helping (Mhelping = 45.2 s; SDhelping = 14.5) than hindering (Mhindering = 28.2 s; SDhindering = 11.2; t (1,17) = 3.458, p = 0.003, d = 1.31, 95% CI [6.638, 27.418]); 14 of the 18 newborns did so (77.7%; p = 0.030, binomial test, two-tailed).
Discussion
The current findings represent evidence that human newborns discriminate different types of social interactions, and that they selectively attend to prosocial interactions over antisocial ones. These findings go significantly beyond past work in lending support to claims that humans are in possession of unlearned mechanisms for detecting and evaluate key features of the sociomoral world20,39,40,41, consistent with theories of the evolution of cooperation14,15,16,17,18,19. Indeed, during their short lives it seems highly unlikely that these newborns could have encountered sufficient relevant inputs to have possibly learned the differences between social approach versus avoidance, and/or helping versus hindering; nor should they have had any basis by which to attribute distinct value to them. Although there is significant evidence for foetal learning of common auditory and gustatory stimuli experienced in the womb66,67, our stimuli are visual and contain information that is entirely unavailable to fetuses. Further, although newborns in the first few days of life are presumably regularly “approached” (moved toward) and “avoided” (moved away from), those actions inevitably lead to both positive and negative outcomes for them including cuddles, feedings, diaper/clothing changes, and heel sticks/other health checks; it is extremely unlikely that such young newborns would have ever either experienced or observed helping and/or hindering in the situation examined here. Finally, newborns demonstrated sensitivity to approach versus avoidance and helping versus hindering between two entirely novel animated faceless agents, but not to the same actions performed by one animated faceless agent toward an identical inert object. These patterns suggest that newborns’ abilities are both abstract and specific enough to distinguish between distinct types of social interaction, significantly extending prior literature on newborns’ predisposition toward social stimuli more generally46,47,48,49,50.
Although the current findings lend significant support to models positing that infants’ prosocial preferences reflect something unlearned68, they nevertheless fail to address the issue of whether those preferences are moral versus social in nature. Indeed, although newborns’ attention to prosocial acts could reflect an innate tuning to the moral world, it is also possible that our newborns merely responded to the social features of the interactions, such as agents’ relative contingency or alignment with other agents, and preferred interactions reflecting greater social alignment25,26,27. Given that relative social alignment (acting with or like others) and relative prosociality (acting for others) are often confounded, particularly within social interactions that can be sufficiently simplified to show to newborns (but see refs. 20,69,70,71 for evidence with older infants), convincingly distinguishing between social versus moral accounts of the current results may prove challenging. That said, we look forward to future work that attempts to distinguish these accounts in newborns, and to work that generalizes these findings beyond a White, Italian newborn sample72.
But how could human newborns, who lack both extensive experience in the socio-moral world and high-level cognitive capacities, demonstrate such abilities? Although the current findings could reflect experience-independent abilities for computational theory of mind73,74, they are also consistent with a growing body of recent work in the behavioural and neural sciences demonstrating apparently high-level social cognitive processing within the visual system itself75. Indeed, the visual system appears sensitive to cues indicating social interactions such as facingness and motion congruency, and even to higher-level social distinctions between roles (e.g., agent versus patient)76 and interaction type and/or valence (e.g., chasing, helping versus hindering)77,78. These capacities appear to be rooted in processing within visual brain areas such as the extrastriate body area (EBA)62 and the posterior superior temporal sulcus (pSTS)79,80, and they have previously been demonstrated in young infants81,82. These findings shed light on a candidate mechanism through which an inexperienced newborn could demonstrate seemingly high-level capacities to distinguish prosocial from antisocial acts. In turn, the findings reported herein strongly suggest that the high-level social abilities recently evidenced by the visual system are unlearned. Finally, future research should continue to examine the extent to which high-level social, and even moral, processing could be triggered by specific socio-visual cues, and so supported by basic features of the visual system.
Methods
This research on newborns was carried out in accordance with the ethical standard of the Declaration of Helsinki and approved by the Research Ethics Committee of the ARNAS Garibaldi Hospital in Catania (protocol number: 146/C.E.; 20/4/2023). Each newborn’s parent gave written informed consent prior to the testing session.
Statistics
Frequentist tests were performed using IBM SPSS Statistics version 29. Kolmogorov-Smirnov tests indicated that data were normally distributed in Experiments 1–3. Levene’s tests confirmed the homogeneity of variances for the test data (approaching/helping vs avoiding/hindering groups in either social and non-social condition, and test trail) of Experiments 1–3.
Experiment 1
Participants
Thirty-six healthy and full-term newborns (18 females; mean age: 5 days; range age: 4–7 days; mean weight: 3180 g) recruited at the Intensive Neonatal Unit of the ARNAS Garibaldi Hospital in Catania were tested after feeding and when they were awake and alert, during their first medical check-up in hospital (from 4 to 8 days of life) after three days of hospitalization. This first medical check-up is performed to monitor weight, bilirubin, and breastfeeding procedures and/or to apply screening for metabolic diseases.
Newborns were White (100%) with Italian parents. Half of the newborns (N = 18) were assigned to the social condition and the other half (N = 18) to the non-social condition. All newborns were full-term (natural birth between 37 and 41 weeks of gestation), with normal APGAR scores (range 8–10) and normal birth weights (range 2500–4200 g). Independent t-tests demonstrated that there were no statistically significant differences between condition in newborns’ weight (t (34) = 0.168, p = 0.867), age in days (t (34) = 0.415, p = 0.680) or gestational age (t (34) = 0.127, p = 0.900). Thirteen additional newborns were tested and excluded from the final sample due to fussiness (n = 2), strabismus (n = 2), crying (n = 2), or a position bias56 (i.e., looking to the right or left side of the screen more than the 85% of the total looking time across both events; ntot = 7; n = 3 in the social condition, and n = 4 in the non-social condition). These exclusion criteria were pre-set and applied prior to any data analysis. Sample size was chosen based on a power analysis using the effect size from previous studies using a similar preferential-looking paradigm with newborns (f = 0.3055,56), and revealed that a total sample size of at least 18 participants per group provides enough power (0.80 with an alpha level of 0.05) to identify a similarly-sized effect. Parental written consent was obtained before testing began.
Apparatus
Participants were tested in a quiet room of the hospital. Newborns were seated on a parent’s lap, 30 cm from the stimulus presentation monitor (24’ screen size, 1920 ×1080-pixel resolution, refresh rates of 75 HZ). Newborns’ visual behaviours were recorded by a camera placed under the monitor to allow offline coding of gaze.
Stimuli
Newborns were randomly assigned to either the social or the non-social condition. In both conditions, newborns were presented with two simple animated events displayed side-by-side, each playing in a simultaneous loop with matched start times and overall timing; one event depicted approach and the other depicted avoidance. In the social condition, approach and avoidance events each involved two grey, faceless self-propelled ball agents, 3 cm in diameter51. In the approach event (Supplementary Movie S1) agent A moved slightly (2.5 cm) toward and then away from agent B twice, and then paused (Fig. 2, panels 1 and 2 left). Agent B then began moving toward agent A, after which agent A began moving toward agent B once again. Agent A eventually reached a position 1.5 cm from Agent B and paused once more, and agent B continued to move toward agent A, eventually stopping next to it (0.70 cm away; Fig. 2, panels 3 and 4, left). The avoidance event (Supplementary Movie S1) began in the same manner as the approach event, with agent A twice moving toward and away from Agent B (Fig. 2, panels 1 and 2, right), Agent B beginning to move toward Agent A, and Agent A moving toward Agent B once again and stopping. Here, however, when Agent B got within 3 cm of Agent A’s final position, Agent B stopped and then backed away, ultimately coming to a stop 6 cm away from Agent A (Fig. 2, panels 3 and 4, right).
Events in the non-social condition were designed to be identical to those in the social condition except for Agent A was replaced by an inert object (also a grey ball) that displayed no self-propelled motion. Thus, only Agent B moved (Supplementary Movie S2). Each event in both conditions lasted 7500 ms (189 frames, 25 frames/second). Movies were produced by looping the animation for a total of 60 s, so newborns saw a total of 8 events.
Procedure
Stimuli presentation was performed using E-Prime 2.0.10 software. Newborns were presented with two trials of approaching and avoiding actions being displayed simultaneously and bilaterally; each trial lasted for 60 seconds. The left/right position of the videos was counterbalanced across participants and flipped between the first and the second trials. At the beginning of each trial, a flickering white cross attention-getter appeared in the centre of the monitor. Each trial began as soon as the newborns looked at the flickering white cross. Parents were instructed not to speak to or otherwise interact with the baby during the experiment.
Coding and reliability
We employed an offline infant-controlled procedure58. Each trial ended when newborns watched each stimulus at least once and shifted their gazes away for more than 10 s. The remaining section was discarded from the looking times analysis.
Newborns’ total fixation time to each side of the screen (i.e., the sum of all fixations) was coded offline from video by a first coder, which was blind to the left-right position of the events, frame by frame and using VirtualDub software (https://www.virtualdub.org).
An additional 50% of the sample was re-coded by a second coder blind to the conditions and the aims of the study. The inter-coder agreements (Pearson correlations) with the second coder of total fixation times were 0.96 for approaching and 0.95 for avoiding actions in the social condition, and 0.90 for approaching and 0.95 for avoiding actions in the non-social condition.
Experiment 2
Participants
Thirty-six healthy and full-term newborns (16 females; mean age: 5 days; age range: 4–7 days; mean weight: 3304 g) recruited at the Intensive Neonatal Unit of the ARNAS Garibaldi Hospital in Catania were tested after feeding and when they were awake and alert, during their first medical check-up, like Experiment 1. They were White (100%) with Italian parents. Half of the newborns (N = 18) were assigned to the social condition and the other half (N = 18) to the non-social condition. All newborns were full-term (natural birth between 37 and 41 gestational weeks), with normal APGAR scores (range 8–10) and normal birth weights (range 2400–4800 g). Independent t-tests demonstrated that there were no statistically significant differences between condition in newborns’ weight (t (34) = 0.122, p = 0.228), age in days (t (34) = 0.206, p = 0.838) or gestational age (t (34) = 1.228, p = 0.900). Nine additional newborns were tested and excluded from the final sample due to fussiness (n = 1) or a position bias (ntot = 8, of which n = 4 in the social condition, and n = 4 in the non-social condition). These exclusion criteria were pre-set and applied prior to any data analysis. Parental written consent was obtained before testing began.
Apparatus
The apparatus was the same used in Experiment 1.
Stimuli
Newborns were randomly assigned to either social or non-social conditions. In both conditions, newborns were presented with two simple animated events displayed side-by-side, each playing in a simultaneous loop; one event depicted helping and the other depicted hindering. The scenario is an adapted version of the hill paradigm used in prior infant studies25,26,27,62.
In the social condition, helping and hindering events each involved two grey, faceless self-propelled ball agents, 3 cm in diameter51. In the helping event (Supplementary Movie S3), agent A starts in the middle of an inclined plane (8 cm total) and agent B starts at the bottom. First, Agent A moves 1.5 cm up toward the top of the plane and then backs down again, twice, as though trying but failing to reach the top (Fig. 3, panels 1 and 2 left). Then, Agent B moves up toward Agent A and contacts it (i.e., it moves 3.5 cm, starting from the bottom to reach the middle of the plane), pushing it up to the top of the hill (they move 3.5 cm together, starting from the middle to reach the up) (Fig. 3 panels 3 and 4, left).
In the hindering event (Supplementary Movie S3), Agent A starts in the middle of the inclined plane and agent B starts at the top. As in the helping event, Agent A twice moves up and down the plane as though trying to reach the top (Fig. 3, panels 1 and 2, right). Then, Agent B moves toward agent A and contacts it, pushing it to the bottom of the hill (Fig. 3, panels 3 and 4, right). Events in the non-social condition were designed to match those in the social condition except for Agent A was replaced by an inert object (also a grey ball) that displayed no self-propelled motion (Supplementary Movie S4), and so made no attempt to climb. Thus, only Agent B moved. Each event in the social conditions lasted 7100 ms (178 frames, 25 frames/s). Movies were produced by looping the animation for a total of 60 s, so newborns saw a total of 8 events. Each event in the non-social condition lasted 6300 ms (158 frames, 25 frames/s), so newborns saw a total of 9 events within 60 s. Events in the non-social condition were slightly shorter (and so looped one more time over 60 s) due to the absence of independent upward movement by the inert ball at the start of each event; notably, motion was matched exactly across distinct events within each condition, and for all conditions was essentially continuous due to the looping nature of the videos.
Procedure
The procedure was the same used in Experiment 1.
Reliability
Coding followed the same criteria as in Experiment 1. Inter-coder agreement (Pearson correlation) with the second coder was 0.95 for both helping and hindering actions in the social condition, and 0.95 for helping and 0.90 for hindering actions in the non-social condition.
Experiment 3
Participants
Eighteen healthy and full-term newborns (8 females; mean age: 5 days; age range: 4–6 days; mean weight: 3060 g) recruited at the Intensive Neonatal Unit of the ARNAS Garibaldi Hospital in Catania were tested, after feeding and when they were awake and alert, during their first medical check-up, like in the previous two experiments. They were White (100%) with Italian parents. All newborns were full-term (natural birth between 37 and 41 gestational weeks), with normal APGAR scores (range 8–10) and normal birth weights (range 2380–4000). Five additional newborns were tested and excluded from the final sample due to sleeping (n = 1), fussiness (n = 1), strabismus (n = 1) and a position bias (n = 2). Parental written consent was obtained before testing began. These exclusion criteria were preregistered (https://osf.io/p84br).
Apparatus, stimuli and procedure
The apparatus was the same used in Experiment 2. Both stimuli and procedure were like those used in Experiment 2, except that newborns were presented with only the social condition.
Reliability
Coding followed the same criteria as Experiments 1 and 2. Inter-coder agreement (Pearson correlation) with the second coder was 0.96 for both helping and hindering actions.
Data analysis
In Experiments 1 and 2, we performed repeated measures ANOVAs of total fixation times with Trial (first vs. second) and Action Type (prosocial vs. antisocial) as within-subject factors, and Condition (social vs. non-social) as a between-subjects factor. Then, follow up planned paired t-tests were used to compare the newborns’ visual attention to the two types of events (prosocial vs antisocial). To explore whether effects emerged at the level of individual newborns, binomial tests were used in each condition comparing the number of infants who looked longer to the more prosocial event (or matched control) to chance. Finally, chi square tests examined whether the patterns of response were different between conditions within each Experiment. In Experiment 3, we performed a repeated measures ANOVA of total fixation times with Trial (first vs. second) and Action Type (prosocial vs. antisocial) as within-subject factors. Then, follow up planned paired t-tests were used to compare the newborns’ visual attention to the two types of events (prosocial vs antisocial), and a binomial test was used to explore individual-level responding.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The raw data generated in Experiments 1–3 are been deposited on the Open Science Framework under the name “Geraci et al. dati”83, and are publicly available at https://doi.org/10.17605/OSF.IO/P84BR. The raw data generated in the validation study on adults are provided as a Source Data file. Source data are provided with this paper.
Code availability
SPSS Statistics Outputs of the analyses are available here: https://doi.org/10.17605/OSF.IO/P84BR.
References
Burkart, J. M., Hrdy, S. B. & Van Schaik, C. P. Cooperative breeding and human cognitive evolution. Evol. Anthropol. 18, 175–186 (2009).
Rand, D. G. & Nowak, M. A. Human cooperation. Trends Cogn. Sci. 17, 413–425 (2013).
Axelrod, R. & Hamilton, W. D. The evolution of cooperation. Sci 211, 1396–1396 (1981).
Melis, A. P. & Semmann, D. How is human cooperation different? Phil. Trans. Roy. Soc. B 365, 2663–2674 (2010).
Tomasello, M., Melis, A. P., Tennie, C., Wyman, E. & Herrmann, E. Two key steps in the evolution of human cooperation: The interdependence hypothesis. Curr. Anthropol. 53, 673–692 (2012).
Tomasello, M. Why we cooperate (MIT Press, 2009).
Hoffman, M. L. Empathy and moral development: implications for caring and justice (Cambridge University Press, 2000).
Decety, J. & Cowell, J. M. Friends or foes: is empathy necessary for moral behavior?. Perspect. Psychol. Sci. 9, 525–537 (2014).
Haidt, J. The emotional dog and its rational tail: a social intuitionist approach to moral judgment. Psychol. Rev. 108, 814 (2001).
Tomasello, M. The role of roles in uniquely human cognition and sociality. J. Theory Soc. Behav. 50, 2–19 (2020).
Singer, P. The expanding circle (Clarendon Press, 1981).
Fehr, E. & Gächter Altruistic punishment in humans. Nature 415, 137–140 (2002).
Balliet, D., Mulder, L. B. & Van Lange, P. A. Reward, punishment, and cooperation: a meta-analysis. Psychol. Bull. 137, 594–615 (2011).
Alexander, R. D. The biology of moral systems (Transaction, 1987).
Cosmides, L., & Tooby, J. Cognitive adaptations for social exchange. In The adapted mind: evolutionary psychology and the generation of culture (eds, Barkow, J., Cosmides, L. & Tooby, J.) 163–228 (Oxford University Press, 1992).
de Waal, F. Primates and philosophers: how morality evolved (Princeton University Press, 2006).
Henrich, J. & Muthukrishna, M. The origins and psychology of human cooperation. Ann. Rev. Psychol. 72, 207–240 (2021).
Joyce, R. The evolution of morality (MIT Press, 2006).
Trivers, R. L. The evolution of reciprocal altruism. Q. Rev. Bio. 46, 35–57 (1971).
Woo, B. M., Tan, E. & Hamlin, J. K. Human morality is based on an early-emerging moral core. Ann. Rev. Dev. Psychol. 4, 41–61 (2022).
Davidov, M. et al. Caring babies: concern for others in distress during infancy. Dev. Sci. 24, e13016 (2021).
Buyukozer Dawkins, M., Sloane, S. & Baillargeon, R. Do infants in the first year of life expect equal resource allocations?. Front. Psychol. 10, 417740 (2019).
Sloane, S., Baillargeon, R. & Premack, D. Do infants have a sense of fairness?. Psychol. Sci.23, 196–204 (2012).
Schmidt, M. F. & Sommerville, J. A. Fairness expectations and altruistic sharing in 15-month-old human infants. PloS One 6, e23223 (2011).
Powell, L. J. & Spelke, E. S. Third-party preferences for imitators in preverbal infants. Open Mind 2, 61–71 (2018).
Spelke, E. S. What babies know: core knowledge and composition, 1 (Oxford University Press, 2022).
Powell, L. J. Adopted utility calculus: origins of a concept of social affiliation. Perspect. Psychol. Sci. 17, 1215–1233 (2022).
Benton, D. T. & Lapan, C. Moral masters or moral apprentices? A connectionist account of sociomoral evaluation in preverbal infants. Cog. Dev., 62, 101164 (2022).
Hamlin, J. K., Wynn, K. & Bloom, P. Three-month-olds show a negativity bias in their social evaluations. Dev. Sci. 13, 923–929 (2010).
Hamlin, J. K. & Wynn, K. Young infants prefer prosocial to antisocial others. Cogn. Dev. 26, 30–39 (2011).
Hamlin, J. K., Wynn, K. & Bloom, P. Social evaluation by preverbal infants. Nature 450, 557–559 (2007).
Geraci, A. & Surian, L. The developmental roots of fairness: infants’ reactions to equal and unequal distributions of resources. Dev. Sci. 14, 1012–1020 (2011).
Geraci, A., Simion, F. & Surian, L. Infants’ intention-based evaluations of distributive actions. J. Exp. Child Psychol. 220, 105429 (2022).
Geraci, A. & Surian, L. Intention-based evaluations of distributive actions by 4-month-olds. Infant Behav. Dev. 70, 101797 (2023).
Lucca, K., Pospisil, J. & Sommerville, J. A. Fairness informs social decision making in infancy. PloS One 13, e0192848 (2018).
Geraci, A., Regolin, L., Simion, F. & Surian, L. Infants’ preferences for approachers over repulsers shift between 4 and 8 months of age. Aggress. Behav. 48, 487–499 (2022).
Kanakogi, Y., Okumura, Y., Inoue, Y., Kitazaki, M. & Itakura, S. Rudimentary sympathy in preverbal infants: preference for others in distress. PloS One 8, e65292 (2013).
Kanakogi, Y. et al. Preverbal infants affirm third-party interventions that protect victims from aggressors. Nat. Hum. Behav. 1, 0037 (2017).
Hamlin, J. K. Moral judgment and action in preverbal infants and toddlers: evidence for an innate moral core. Curr. Dir. Psychol. Sci. 22, 186–193 (2013).
Wynn, K., Bloom, P., Jordan, A., Marshall, J. & Sheskin, M. Not noble savages after all: Limits to early altruism. Curr. Dir. Psychol. Sci. 27, 3–8 (2018).
Dawkins, M. B., Ting, F., Stavans, M. & Baillargeon, R. Early moral cognition: a principle-based approach, pp. 7–16. (MIT Press, 2020).
Cushman, F., Kumar, V. & Railton, P. Moral learning: Psychological and philosophical perspectives. Cognition 167, 1–10 (2017).
Sagi, A. & Hoffman, M. L. Empathic distress in the newborn. Dev. Psychol. 12, 175 (1976).
Dondi, M., Simion, F. & Caltran, G. Can newborns discriminate between their own cry and the cry of another newborn infant?. Dev. Psychol. 35, 418 (1999).
Ruffman, T., Lorimer, B. & Scarf, D. Do infants really experience emotional contagion?. Child Dev. Perspect. 11, 270–274 (2017).
Farroni, T. et al. Newborns’ preference for face-relevant stimuli: effects of contrast polarity. Proc. Natl Acad. Sci. 102, 17245–17250 (2005).
Johnson, M. H., Dziurawiec, S., Ellis, H. & Morton, J. Newborns’ preferential tracking of face-like stimuli and its subsequent decline. Cognition 40, 1–19 (1991).
Turati, C., Bulf, H. & Simion, F. Newborns’ face recognition over changes in viewpoint. Cognition 106, 1300–1321 (2008).
Valenza, E., Simion, F., Cassia, V. M. & Umiltà, C. Face preference at birth. J. Exp. Psychol. Hum. Percept. Perform. 22, 892–903 (1996).
Farroni, T., Menon, E. & Johnson, M. H. Factors influencing newborns’ preference for faces with eye contact. J. Exp. Child Psychol. 95, 298–308 (2006).
Simion, F., Regolin, L. & Bulf, H. A predisposition for biological motion in the newborn baby. Proc. Natl Acad. Sci. 105, 809–813 (2008).
Craighero, L., Lunghi, M., Leo, I., Ghirardi, V. & Simion, F. Newborns’ attention is driven by the translational movement. Vis. Cogn. 24, 487–498 (2016). 2016.
Craighero, L., Ghirardi, V., Lunghi, M., Panin, F. & Simion, F. Two-day-old newborns learn to discriminate accelerated-decelerated biological kinematics from constant velocity motion. Cognition 195, 104126 (2020).
Di Giorgio, E., Lunghi, M., Vallortigara, G. & Simion, F. Newborns’ sensitivity to speed changes as a building block for animacy perception. Sci. Rep. 11, 542 (2021).
Craighero, L., Leo, I., Umiltà, C. & Simion, F. Newborns’ preference for goal-directed actions. Cognition 120, 26–32 (2011).
Addabbo, M., Roberti, E., Colombo, L., Picciolini, O. & Turati, C. Newborns’ early attuning to hand-to-mouth coordinated actions. Dev. Sci. 25, e13162 (2022).
Longhi, E. et al. Discrimination of biomechanically possible and impossible hand movements at birth. Child Dev. 86, 632–641 (2015).
Filippetti, M. L., Johnson, M. H., Lloyd-Fox, S., Dragovic, D. & Farroni, T. Body perception in newborns. Curr. Biol. 23, 2413–2416 (2013).
Roberti, E., Addabbo, M., Colombo, L., Porro, M. & Turati, C. Newborns’ perception of approach and withdrawal from biological movement: a closeness story. Infancy 29, 22–30 (2024).
Papeo, L. & Abassi, E. Seeing social events: The visual specialization for dyadic human–human interactions. J. Exp. Psychol. Hum. Percept. Perform. 45, 877 (2019).
Abassi, E., & Papeo, L. Category-selective representation of relationships in the visual cortex. J. Neurosci. 44, e0250232023 (2024).
Gandolfo, M. et al. Converging evidence that left extrastriate body area supports visual sensitivity to social interactions. Curr. Biol. 34, 343–351 (2024).
Zanon, M., Lemaire, B. S., Papeo, L. & Vallortigara, G. Innate sensitivity to face-to-face biological motion. iScience, 27, 108793 (2024).
De Roni, P., Geraci, A., Simion, F. & Regolin, L. Sensitivity to the role of an animated agent from observed interactions in newborn chicks (Gallus gallus). R. Soc. Open Sci. 10, 210020 (2023).
Kuhlmeier, V., Wynn, K. & Bloom, P. Attribution of dispositional states by 12-month-olds. Psychol. Sci. 14, 402–408 (2003).
Gervain, J. The role of prenatal experience in language development. Curr. Opin. Behav. Sci. 21, 62–67 (2018).
Ventura, A. K. & Worobey, J. Early influences on the development of food preferences. Curr. Biol. 23, R401–R408 (2013).
Hamlin, J. K. Core morality? Or merely core agents and social beings? A response to Spelke’s what babies know. Mind Lang. 38, 1323–1335 (2023).
Woo, B. M. & Spelke, E. S. Toddlers’ social evaluations of agents who act on false beliefs. Dev. Sci. 26, e13314 (2023).
Woo, B. M. & Spelke, E. S. Infants and toddlers leverage their understanding of action goals to evaluate agents who help others. Child Dev. 94, 734–751 (2023).
Woo, B. M., Liu, S., Gweon, H. & Spelke, E. S. Toddlers prefer agents who help those facing harder tasks. Open Mind 8, 483–499 (2024).
Lucca, K. et al. Infants’ social evaluation of helpers and hinderers: a large-scale, multi-lab, coordinated replication study. Dev. Sci. 28, e13581 (2025).
Baker, C. L., Jara-Ettinger, J., Saxe, R. & Tenenbaum, J. B. Rational quantitative attribution of beliefs, desires and percepts in human mentalizing. Nat. Hum. Behav. 1, 0064 (2017).
Leslie, A. M., Friedman, O. & German, T. P. Core mechanisms in ‘theory of mind’. Trends Cogn. Sci. 8, 528–533 (2004).
McMahon, M. & Isik, L. Seeing social interaction. Trends Cogn. Sci. 27, 1165–1179 (2023).
Vettori, S., Odin, C., Hochmann, J. R. & Papeo, L. A perceptual cue-based mechanism for automatic assignment of thematic agent and patient roles. J. Exp. Psychol. Gen. 154, 787–798 (2024).
Gao, T., Newman, G. E. & Scholl, B. J. The psychophysics of chasing: a case study in the perception of animacy. Cogn. Psychol. 59, 154–179 (2009).
Isik, L., Koldewyn, K., Beeler, D. & Kanwisher, N. Perceiving social interactions in the posterior superior temporal sulcus. Proc. Natl. Acad. Sci. 114, E9145–E9152 (2017).
Pitcher, D. & Ungerleider, L. G. Evidence for a third visual pathway specialized for social perception. Trends Cogn. Sci. 25, 100–110 (2021).
Brady, R. G. et al. Newborn brain function and early emerging callous-unemotional traits. JAMA Psychiatry 81, 303–311 (2024).
Tauzin, T. & Gergely, G. Co-dependency of exchanged behaviors is a cue for agency attribution in 10-month-olds. Sci. Rep. 11, 18217 (2021).
Schlottmann, A., Ray, E. & Surian, L. Emerging perception of causality in action-and-reaction sequences from 4 to 6 months of age. J. Exp. Child Psychol. 112, 208–230 (2012).
Geraci, A., Surian, L. & Hamlin, K. Newborns’ sensitivity to social goals. Open Science Framework, https://doi.org/10.17605/OSF.IO/P84BR (2024).
Acknowledgements
We are grateful to the General, Medical and Administrative Directors of the ARNAS Garibaldi Hospital for letting this research. We thank the Director of Maternal and Infant Department of the ARNAS Garibaldi Hospital for his precious support. Special thanks to all medical and nursing staff of the Intensive Neonatal Unit of the ARNAS Garibaldi Hospital, to parents and newborns for their excellent collaboration. We are very grateful to Daniel Loria and Emanuela Borzì for their help in collecting and coding data. We would like to thank Prof. Francesca Simion for her valuable teachings about research on newborns.
Ethics declarations
Competing interests
The authors declare no competing of interests.
Peer review
Peer review information
Nature Communications thanks Elisa Roberti, Chiara Turati, Ermanno Quadrelli and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Geraci, A., Surian, L., Tina, L.G. et al. Human newborns spontaneously attend to prosocial interactions. Nat Commun 16, 6304 (2025). https://doi.org/10.1038/s41467-025-61517-3
Received: 09 June 2024
Accepted: 24 June 2025
Published: 08 July 2025
DOI: https://doi.org/10.1038/s41467-025-61517-3
.png)