Introduction
Memory storage is a dynamic process shaped by time-dependent maturation mechanisms and reactivation cues, whether internal or external1. Research during both wakefulness and sleep suggests that neural activity patterns present at encoding are reactivated post-acquisition, facilitating memory stabilization and maintenance—a process known as consolidation2,3,4,5. Thus, reactivation is a prerequisite for past experiences to influence our present and guide decision-making. Notably, the reactivation that leads to memory retrieval can also transform the memory itself6,7. Retrieving recently acquired information within the consolidation window can strengthen, inhibit, or update the target memory8,9. For nearly a century, research has shown that retrieval practice—the “testing effect”—enhances long-term retention across various types of information, from text passages to visuospatial locations10,11,12,13,14.
The testing effect suggests that retrieval strengthens the memory of target items and enhances associated non-retrieved items by reactivating both the items and their episodic context (i.e., retrieval-induced facilitation15). Prior studies have demonstrated that strengthening retrieved items can indirectly benefit related (contextually or semantically) or even unrelated information, as well as the spatial and temporal context in which learning occurs13,16. For example, retrieval of educational material enhances the retention of related but untested items15, and retrieval of individual words from a list can improve recall of unpracticed words17. Similarly, retrieval practice can strengthen the memory of not only the tested information but also contextually linked details, likely through neural reactivation18.
Previous research suggests that while restudying primarily benefits the reviewed material, retrieval practice activates contextually and semantically related elements19. This effect is more pronounced when there is a longer interval before the final test, likely due to consolidation processes during sleep8. However, retrieval interventions can have mixed effects on related information20. On one hand, the inhibition hypothesis posits that non-retrieved information during reactivation is suppressed, hindering its later access (Retrieval-Induced Forgetting)21. On the other hand, the facilitation hypothesis suggests that by strengthening tested elements, the original context is reactivated, which can benefit non-reactivated elements as well (Retrieval-Induced Facilitation)22. The extent of these processes appears to depend on whether the retrieval intervention occurs in a context similar to or different from the original learning context20.
Most studies focus on reactivation interventions applied before consolidation10,23. However, retrieving a consolidated memory can trigger reconsolidation—a process in which the memory becomes temporarily unstable before being restabilized, potentially allowing for modifications in its strength or content12,24. Multiple studies have shown that presenting reminders (reactivation by showing a fragment of a previously studied item) of information learned one or several days prior enhances memory precision and retention and protects against interference25. Further, reactivation of a consolidated memory followed by new learning allows for the integration of new information into existing memories, revealing the process of memory updating induced by reconsolidation12. Thus, for example, the presentation of new information post-reactivation may be intrusive and weaken the original episodic memory (interference) or be integrated without altering the strength or expression of the original memory26. Research on animals and humans has proposed the notion of reactivation specificity, suggesting that only the reactivated elements post-consolidation can be modified through behavioral or pharmacological interventions4. However, other studies27,28,29 in animal fear-conditioning indicate that strengthening through reactivation and reconsolidation may also extend to non-reactivated items. For instance, Liu et al.28 found that using independent predictors of an aversive stimulus during reactivation could trigger the reconsolidation of the entire associated memory. Similarly, Debiec et al.29 demonstrated that reactivating one element of a compound stimulus (e.g., a tone) could initiate reconsolidation of the entire memory if the compound was presented during acquisition. Some authors have proposed that not all reminders trigger reconsolidation, but rather that it depends on the occurrence of a prediction error—defined as a discrepancy between expected and actual outcomes—which allows for the reactivation of the memory and its subsequent modification30,31. However, empirical support for the role of prediction error in reconsolidation remains mixed32,33, particularly in humans (but see refs. 30,34,35), prompting alternative explanations such as the testing effect or behavioral tagging36.
Episodic memory reflects our capacity to acquire, consolidate, and retrieve complex, multimodal, and contextualized events37,38. It is believed that events are stored in episodic memory as coherent representations within modular networks, where constituent elements are interconnected such that a cue can trigger the reinstatement (“re-experiencing”) of these elements and their acquisition context through pattern completion in the hippocampus (holistic retrieval39,40,41,42,43). Context encompasses various aspects of an episode that integrate central and peripheral elements into a cohesive unit44,45. Thus, context can refer to the location of an event, its temporal order, or its semantic space. Given that episodic memories tend to exhibit coherence and retrieval is often holistic46, we anticipate that the direct reactivation of a consolidated target memory will primarily strengthen that target memory. Furthermore, due to the interconnected nature of episodic memory, reactivation will also indirectly reactivate and strengthen associated information from the original acquisition context43. We aim to explore how reactivation might enhance the encoding of concurrently presented information, even if unrelated to the target memory. Specifically, we define “direct” reactivation as the retrieval of the target item and “indirect” reactivation as the activation of associated elements through contextual retrieval or integration processes. During retrieval, the reactivation of target items can directly improve their retention while indirectly activating other items from the acquisition context through holistic retrieval processes. Therefore, our central hypothesis is that direct reactivation of episodic memory strengthens both the target items and, indirectly, those associated with their original context. We propose that this indirect strengthening effect can operate retroactively, enhancing previously consolidated information acquired in the same context as the target memory. We also consider the possibility of a proactive effect on the integration of new information presented during reactivation. Thus, reactivation may retroactively strengthen consolidated information or facilitate the integration of new information into the memory system through shared spatiotemporal or contextual features. To investigate this hypothesis, we conducted four experiments with young adults over three consecutive days (n = 238). On Day 1, participants underwent paired-associate (face-name) memory training, followed by either target memory reactivation or a control reactivation 24 h later. Memory performance was assessed on Day 3. To explore the indirect effects of reactivation, participants performed a decision task involving everyday objects (peripheral memory) either during target memory acquisition on Day 1 (same context) or concurrently during reactivation on Day 2 (new context) and tested on Day 3. Finally, to examine the dependency of direct and indirect effects on the shared context, we conducted two control experiments where peripheral memory acquisition occurred in a context separate from either the acquisition of the paired associates on Day 1 (n = 57) or their reactivation on Day 2 (n = 51).
Experiment 1
Materials and methods
There was no preregistration of this study. Experiment 1 was intended to assess how direct reactivation of a consolidated target memory can directly strengthen this reactivated information and indirectly strengthen a non-reactivated memory associated with the original acquisition context. Participants performed an experiment over three days separated by 24 h. On Day 1, participants acquired a target memory (paired associates) and performed a decision-making task (object classification) to acquire the peripheral memory (Fig. 1) incidentally. Importantly, participants were unaware that their memory for the objects (peripheral memory) presented during the decision task would be tested later. On Day 2, a group received a Reactivation Intervention (RI), and another group received a control Reactivation (RC). On Day 3, the peripheral memory was assessed first, followed by the target memory.
a Day 1—Memory acquisition. First Trial: The study round presented all face-name pairs with all the names. Training trials 1–3: Each face was shown, and participants had to type the corresponding name after a sound and visual cue. Two unique objects were presented after each face. b Day 2—Memory reactivation: RI used incomplete reminders to reactivate memory. Each face was briefly shown with a name cue and an interruption message. RC was similar but omitted the name cue. c Day 3—Memory Evaluation. Object recognition (Peripheral memory) tested the recognition of old and new objects. Memory for face-name pairs (Target memory) was tested across three trials. d Summary of variations between experiments. “Target” refers to the acquisition and training of face-name associative pairs, “Peripheral” refers to the judgment task on the objects, “R” refers to both types of reactivations, “AM questions” refers to the autobiographical questions task, “Peripheral + Target Testing” refers to the object recognition task and the name recall evaluation.
Participants
Participants were recruited via social media and university channels. A total of 81 participants participated in this Experiment. Participants who responded correctly to 70% of the target memory during the last trial on Day 1 were included in the analysis. The final sample comprised 61 individuals from Argentina (age M = 25.5, SD = 3.47; 31 women and 30 men). The sample size was based on a power analysis of our previous memory strengthening studies25 and Monte Carlo simulations using the R package simr, targeting the interaction between group and block (minimum effect size of 0.5 with 80% power, with confidence intervals above the 95% level). All experiments were performed online on the Gorilla Experiment Builder47. Given that the initial experiments were conducted during the COVID-19 pandemic, we included the Positive Affect Negative Affect Scale (PANAS) to ensure that participants’ affective states did not differ significantly from those observed in pre-pandemic conditions using national norms48. Numerous studies conducted during this period have reported an increase in symptoms of anxiety, depression, and stress, which may have adversely impacted cognitive task performance49. Our results confirmed that PANAS scores were consistent with typical pre-pandemic values. Overall, participants expressed more positive (PANAS, M = 31.7, 95% CI [30.28, 33.24]) than negative general affect (PANAS, M = 16.15, 95% CI [14.67, 17.63], F (1.138) = 218.265, P < 0.001, ηp2 = 0.62), and there was no conclusive evidence of differences between groups (F (1.138) = 1.022, P = 0.31, ηp2 = 0.007, BF01 = 0.93).
Stimuli
Images of 12 faces from the FACES repository (Max Planck Society50), were used as target items in the face-name task. Images from individuals in the approximate age range of the participants with neutral expressions were selected, half men and the other half women. The names assigned were extracted from the National Registry of Persons (Argentinian Civil Registry), and three-syllable names were selected from the most common ones between 1980 and 2000 (e.g., “Benjamin”). The chosen names and faces were randomly paired, respecting the corresponding genders, and these associations were maintained for all participants. For the decision task, which included the peripheral items, 144 everyday objects were selected from the Mnemonic Similarity Task database51, which contains images of everyday objects. Seventy-two objects were randomly assigned to the decision task on Day 1 and were presented as “Old” during peripheral memory recognition on Day 3. An additional 72 objects were used as “New” during testing on Day 3. Since the participants had to decide whether their use mainly was Indoors or Outdoors, objects with a significantly higher frequency of use in one of the two scenarios were selected (i.e., a tractor was primarily considered outdoors, and television was considered mostly indoors). The final sample had a distribution of 50% Indoor and 50% Outdoor objects.
Procedure
Before the experiment, participants completed the PANAS52 and signed an informed consent form approved by the Ethics Committee of the Instituto de Investigaciones Médicas Alfredo Lanari. Moreover, participants were asked to report their gender in a multiple-choice question including Woman, Man, Non-Binary participants, “Other” (specify), and “Prefer not to say”).
Day 1 (target memory acquisition and decision task)
Participants received instructions to acquire 12 face-name associations (target memory) in four trials interspersed with a simple decision-making (object categorization) task, which later served as peripheral memory (Fig. 1a). In the first trial, all the face-name pairs were presented in the center of the screen with the corresponding names for 3 s. Training trials 1–3 consisted of the face presentation for 1 s, then the name´s first syllable appeared on the top of the screen for 1.75 s, followed by a sound cue (1 s), which signaled that the participant was allowed to respond with the keyboard and complete the entire name. Participants performed a decision task between each presentation of the paired associates, categorizing everyday objects as primarily indoor or outdoor. After a blank screen of 0.5 s, the object appeared accompanied by an “Indoors (I)” and “Outdoors (O)” legend. The indication was to press the initial letter to inform the most common place of use. Irrespective of when they pressed the key, each object was shown for 3 s, separated by a 0.5 s blank screen. Participants categorized two objects for each paired-associated presentation. Each object was presented a single time without repetition, yielding a total of 72 unique objects shown.
Day 2 (target memory reactivation)
Participants were randomly assigned to either a Reactivation Intervention (RI) or a Reactivation Control (RC; Fig. 1b). All participants were instructed to perform the same face-name task as on Day 1, which involved completing the full name of the person when prompted by the response cue and sound. Importantly, participants were not explicitly informed that the objects (peripheral memory) presented on Day 1 would not be shown or mentioned on this day.
Reactivation intervention (RI): Based on our previous work and sleep studies53,54, we constructed incomplete reminders to reactivate the consolidated target memory acquired on Day 1 and indirectly its associated peripheral memory (objects). This type of reactivation session was demonstrated to have superior effects on episodic memory retention than other types of reactivation25 and is similar to those used to investigate the testing effect55. The reactivation session consisted of the presentation of each face for 2 s, followed by the name´s first syllable for 1.75 s and an interruption message (“Trial interrupted”) without the sound cue that indicated the participant to start writing on Day 1. Two reactivation trials for each target item were used.
Reactivation control (RC): The procedure for the reactivation control was equivalent to the reactivation intervention, except that the name´s first syllable was not presented, resulting in a presentation of each face for a total of 3.75 s. This type of intervention is considered to have a lesser impact on memory performance compared to the RI25. Previous studies that have used similar reactivations to the RC56,57 indicate that this procedure does not alter long-term memory retention to the same degree as RI30, as the degree of prediction error would be insufficient with respect to the target reactivation. We used this intervention to control the reactivation itself and explore the specificity of the reactivation intervention.
Participants could not respond or complete the name in any type of reactivation.
Day 3 (memory evaluation)
On Day 3, a surprise evaluation of the peripheral memory was performed, and the target memory was tested separately (Fig. 1c).
Peripheral memory recognition (Objects): the 72 “Old” objects from Day 1 were presented with 72 “New” objects that participants had not seen during the experiment. A total of 144 objects were shown in a completely randomized manner for each participant. They were asked to report whether the object displayed on the screen was “New” or “Old” to indicate whether they had seen it on Day 1. Participants were also instructed to respond as quickly as possible, and reaction time (RT) for each presented object was recorded.
Target memory (face-name pairs): Memory retention was assessed across three testing trials (ts1, ts2, ts3) on Day 3, where “ts” denotes “testing session”. Testing was similar to the training task on Day 1, except that the first syllable was not presented (uncued). Thus, participants observed the face in the center of the screen for 2.5 s, followed by the 1-s auditory signal and written sign indicating to write the name. If participants pressed the enter key after writing the name, or if 7 s elapsed, they received feedback on the corresponding name. The inter-item duration was the same as on Day 1.
Analytic strategy
Data analysis was implemented in R 4.3.158 using lme4 and emmeans packages. Generalized Mixed-effects (Hierarchical) regression models were used for the Target and Peripheral memory analysis. Mixed-effects models allow for the inclusion of both fixed and random effects, accommodating various types of response variables and handling complex data structures such as nested or hierarchical designs. Data distribution was assumed to be normal but this was not formally tested.
Target memory accuracy was modeled using a logistic regression model. The model included fixed effects for reactivation type (Reactivation Intervention vs. Reactivation Control), Trial, and their interaction. Participant ID and stimulus were included as random effects. The model formula was: Response (Correct/Incorrect) ~ Trial * Reactivation (RI/RC) + (1| Participant) + (1| stimulus).
We adopted a signal detection approach (SDT) to analyze the recognition of the peripheral memory. We used a mixed-effects probit model59 to analyze response bias (c) and Sensitivity (d’) during the object recognition task60. SDT takes advantage of the precision in modeling the decision-making process during recognition (Old/New), as well as its ability to control for initial biases (c) during recognition and infer participants’ general ability to discriminate between item conditions (Old/New, Sensitivity d´). The model predicted “Old” vs. “New” responses based on Item Condition (Old vs. New), Reactivation (RI vs. RC), and random effects for Participant and Item. The random effects controlled for individual differences in overall responding and item-specific variability. A random slope for Item Condition by Participant allowed for individual differences in how participants differentiated between Old and New items. The statistical model was: Recognition (“saying Old”) ~ Item Condition (Old/New) * Reactivation (RI/RC) + (1+ Item Condition | Participant) + (1|Object). Effect coding contrasts (−1/2; 1/2) were used to facilitate interpretation of the results. In this SDT implementation, the intercept corresponds to the bias (c), the Item Condition to the overall Sensitivity, and the Reactivation x Item Condition interaction to the effect of the group on Sensitivity. We also estimated individual subject sensitivity (d’) based on the fitted model. To achieve this, we generated predicted probabilities of “Old” responses for each participant in each Item Condition (Old/New) on the probit scale, ensuring these predictions incorporated the random effects structure of the model to provide participant-specific estimates. Finally, we calculated the Sensitivity (d’) for each participant as the difference between the predicted probit values for the two item conditions. Additionally, we analyzed the total recognition accuracy (Old + New) of correct responses during Peripheral memory evaluation using a mixed-effects logistic regression. Reactivation type and Item Condition were considered fixed effects, and each participant and object were included as random effects. The statistical model consisted of: Response (Correct/Incorrect) ~ Item Condition (Old/New) * Reactivation (RI /RC) + (1 | Participant) + (1| Object). In summary, our analysis focused on two complementary memory metrics: sensitivity (derived from SDT analysis), which provides a robust measure of the core cognitive ability being studied (discrimination), and accuracy, which offers a complementary, general view of performance. Finally, Reaction times (RT) were measured as an additional assessment of the strength of memory retrieval. Previous studies have shown that stronger memories require shorter access times, as reflected in faster reaction times11. RT during object recognition were evaluated using similar linear models, with the main difference being that the response variable in this case was continuous. RT was normalized using a logarithmic transformation before analysis. The formula was: Log(RT) ~ Item Condition (Old/New) * Reactivation (RI/RC) + (1/Participant) + (1| Object). In all cases, the significance of fixed factors was assessed using the likelihood-ratio test (ANOVA command in R) using Satterthwaite’s approximation to degrees of freedom. We calculated Marginal R2 (variance explained by fixed factors) and Conditional R2 (variance explained by the full model, including both fixed and random effects) to quantify the effect sizes. Recognition trials with response times shorter than 200 ms or longer than 2.5 s were excluded from all analyses. The inclusion of “item” as a random effect was hypothesized to account for variability in memory performance attributable to differences in how individual stimuli were processed. Although all stimuli were selected from a standardized database, inherent differences in salience, distinctiveness, or difficulty could influence memory performance. By modeling “item” as a random effect, we aimed to control for these potential item-specific variations, ensuring that our results more accurately reflect effects driven by experimental conditions rather than stimulus identity. Furthermore, model comparison using the Akaike Information Criterion (AIC) indicated a better fit for the model, including “item” as a random effect, supporting its inclusion as a data-driven decision. Lastly, to quantify the evidence for or against the null hypothesis, we calculated Bayes Factors (BF01) based on the Bayesian Information Criterion (BIC) in R and JASP when appropriate. This approach compares the likelihood of the data under two models, typically a full model (i.e., including an interaction term) and a reduced model (i.e., excluding the interaction term), while penalizing model complexity. The resulting BF01 indicates the relative evidence for the reduced model compared to the full model. The following interpretation criteria were used for BF01 values: a value less than 1 indicates that both models are equally likely, a value greater than 3 suggests moderate evidence in favor of the null model, and a value greater than 10 provides strong evidence in favor of the null model. This methodology allowed for more robust inferences about the absence of an effect, by quantifying the evidence in favor of the null hypothesis.
Results
Target memory (paired-associates) acquisition and evaluation
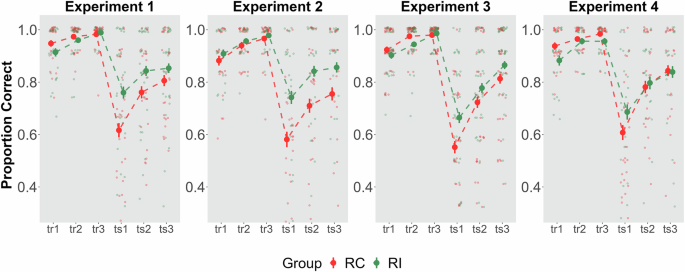
As depicted in Fig. 2, Target memory (face-name pairs) training was similar between groups and different according to the type of reactivation received on Day 2. During the beginning of the training session, all groups started from a similar retention level (tr1 RI vs. RC, β = 0.72, 95% CI [0.09, 1.35]; P = 0.47, BF01 = 4.2) and, at the end of Day 1, participants responded correctly at least 70% of the paired associates (tr3 RI vs RC, β = 0.66, 95% CI [−0.73, 2.05], P = 0.35, BF01 = 3.88).
As expected, target memory retention on Day 3 was consistently different depending on the reactivation type (Main effects: Reactivation X2 (1) = 3.5191, P = 0.06, Trial X2 (5) = 280.7524, P < 0.0001, Reactivation × Trial Interaction, X2 (5) = 13.9077, P = 0.01, R2 Conditional = 0.48, R2 Marginal = 0.30). More specifically for Experiment 1, the group that received the Reactivation Intervention (RI) had better memory performance on the paired-associates task in the first testing trial on Day 3 (ts1 contrast RI vs. RC: β = 2.50, 95% CI [1.78, 3.22], P = 0.008) task than the group receiving the Reactivation Control (RC). Overall, these results confirm that RI can directly reactivate and strengthen the consolidated Target Memory compared to RC.
Peripheral memory (object recognition)
Overall, participants showed a bias to classify objects as novel (bias c: β = −0.57, 95% CI [−0.60, −0.41], P < 0.001, Marginal R2/Conditional R2 = 0.39/0.49) but could reliably discriminate Old from New objects (sensitivity d´: β Item Condition = 1.84, 95% CI [1.74, 1.92], P < 0.001). Old responses were similar for both reactivation interventions, indicating weak evidence for a group effect on bias (Reactivation β = 0.20, 95% CI [−0.02, 0.38], P = 0.10, BF01 = 1.28). More importantly, the analysis of the interaction between sensitivity and Reactivation revealed that the RI group discriminated objects better than RC (Fig. 3, MRI d´ = 1.85, 95% CI [1.75, 1.93], MRC d´ = 1.56, 95% CI [1.49, 1.67], MDifference = −0.290 [−0.38, −0.14], Cohen´s d = −0.19; Reactivation × sensitivity d´: β = −0.28, 95% CI [−0.40, −0.14], P < 0.001). There was weak evidence for differences in recognition accuracy between reactivation groups (Fig. 3, MRI = 0.77, 95% CI [0.76, 0.79], MRC = 0.75, 95% CI [0.73, 0.76], Main effects: Reactivation X2 (1) = 0.99, P = 0.31, BF01 = 2.4, Item Condition X2 (1) = 172.70, P < 0.0001, Marginal R2/Conditional R2 = 0.22/0.39). However, the RI group had higher peripheral memory accuracy than the RC group for New objects (Reactivation x Condition Interaction X2 (1) = 7.41, P < 0.0001, MRI = 0.92, 95% CI [0.90, 0.93], MRC = 0.87, 95% CI [0.86, 0.89], z = 2.22, P = 0.015) but this was not the case for Old ones (MRI = 0.64, 95% CI [0.62, 0.66], MRC = 0.62, 95% CI [0.60, 0.64], z = 0.38, P = 0.70, BF01 = 3.74).
a Estimated fitted means with SE across Experiments and Reactivation type. Smaller points show individual performance. Groups that acquired both face-name pairs and objects jointly (Experiments 1 and 3) and received the Reactivation Intervention (RI) showed higher sensibility and accuracy than the Reactivation Control (RC) groups. The groups that acquired the objects separately exhibited greater sensitivity and accuracy than those that acquired them collectively, regardless of reactivation treatment. b Table showing Sensitivity (d-prime) and bias (c) for each group in each experiment.
Reaction Times (RT) analysis during peripheral memory recognition revealed no significant interaction between Reactivation and Item Condition (β = 0.001, 95% CI [−0.02, 0.02], P = 0.90, BF01 > 2000; Reactivation Main effect β = −0.002, 95% CI [−0.08, 0.08], P = 0.95; Marginal R2/Conditional R2 = 0.007/0.22). Finally, there was a significant main effect of Item Condition (β = 0.05, 95% CI [0.03, 0.07], P < 0.0001), indicating that RT was significantly longer for Old objects compared to New ones. The results of Experiment 1 suggest that a robust reactivation (RI) of a consolidated target memory directly strengthens the target memory and indirectly enhances the associated peripheral objects that share the initial acquisition context.
Experiment 2
Materials and methods
Experiment 2 aimed to assess how a separated acquisition context for target and peripheral memories can limit the indirect strengthening effect of a non-directly reactivated memory. For this purpose, participants completed a task similar to the previous experiment, using the same paired associates and everyday objects for the peripheral memory task. However, unlike Experiment 1, these two sets of stimuli were acquired in different contexts. On Day 1, after target memory acquisition, participants completed a 10 min. distracting autobiographical task and then categorized objects in the decision task, which was designed to represent a distinct context from the paired-associates task by altering the typography, font color, and background of the presented images. The autobiographical task was intended to separate the Target and Peripheral memory acquisition contexts by creating a different context and focusing on a personal “retrieval mode”61. This task consisted of a series of questions about typical events in a person’s life, and participants were asked to remember the first and the last time they went through these events. For example, participants were asked about the last time they practiced a sport or the first time they went to the movies. Finally, for each question, they were asked to think in more specific details of the event, like “Do you remember who you were with?” or “Do you remember how you felt at the time?”. On Day 2, groups received the RI or RC, and on Day 3, peripheral memory was assessed first and then the target memory. A total of 57 participants participated in this study. The same exclusion criteria and analytic strategy as Experiment 1 were applied. The final sample comprised 52 individuals (age M = 23.3, SD = 4.09; 31 women, 21 men). Participants exhibited a predominance of positive affect (PANAS M = 33.00, 95% CI [31.24, 34.7]) in relation to negative affect (PANAS M = 16.64, 95% CI [14.88, 18.40], F (1.115) = 7606.195, P < 0.001, ηp2 = 0.60), with ambiguous evidence for differences between the groups (F (1.115) = 1.446, P = 0.24, ηp2 = 0.01, BF01 = 0.88).
Results
Target memory (paired-associates) acquisition and evaluation
Consistent with the findings from Experiment 1, Day 1 memory accuracy for the Target memory (face-name) training showed modest evidence for differences between groups (Fig. 2, tr1 RI vs RC contrast β = 1.34, 95% CI [0.19, 2.48], P = 0.50, BF01 = 3.6; tr3 RI vs RC, β = 0.38, 95% CI [−0.80, 1.57], P = 0.52, BF01 = 4.07). However, accuracy on Day 3 varied as a function of the reactivation type received (Main effects: Reactivation X2 (1) = 4.8089, P = 0.02, Trial X2 (5) = 274.9899, P < 0.0001; Reactivation x Trial Interaction, X2 (5) = 5.8805, P = 0.31, R2 Marginal = 0.22, R2 Conditional = 0.45). Specifically, groups receiving RI on Day 2 demonstrated significantly better performance on the first testing trial of the paired-associates task of Day 3 (ts1 contrast RI vs RC, β = 2.56, 95% CI [0.65, 4.46], P = 0.01).
Peripheral memory (object recognition)
Similar to Experiment 1, participants displayed a bias towards classifying objects as novel (bias c: β = −0.44, 95% CI [−0.54, −0.34], P < 0.001, Marginal R2/Conditional R2 = 0.48/0.53) and had a reliable sensitivity to discriminate between Old and New items (sensitivity d´: β = 2.00, 95% CI [1.92, 2.08], P < 0.001). Interestingly, the reactivation intervention did not significantly affect peripheral memory recognition (Fig. 3, MRI d´ = 2.08, 95% CI [1.97, 2.19], MRC d´ = 1.93, 95% CI [1.82, 2.05], MDifference = −0.150 [−0.30, 0.01], Cohen´s d = −0.08; Reactivation × sensitivity d´: β = −0.13, 95% CI [−0.32, −0.008], P = 0.09, BF01 = 3.2; Reactivation β = 0.04, 95% CI [−0.14, 0.24], P = 0.64, BF01 = 11.1). Moreover, there was weak evidence of differences in recognition accuracy between Reactivation groups (Fig. 3, MRI = 0.81, 95% CI [0.80, 0.83], MRC = 0.80, 95% CI [0.79, 0.81], Main effects: Reactivation X2 (1) = 0.41, P = 0.52; BF01 = 1.8, Item Condition X2 (1) = 104.02, P < 0.001), but strong evidence of no interaction with Item Condition (Reactivation × Condition X2 (1) = 1.60, P = 0.20, BF01 = 10.3, Marginal R2/Conditional R2 = 0.18/0.38). Finally, the reactivation intervention did not significantly affect RT during peripheral memory recognition (Item Condition β = 0.03, 95% CI [0.01, 0.05], P < 0.001; Reactivation β = −0.008, 95% CI [−0.08, 0.07], P = 0.84; Reactivation x Condition β = −0.002, 95% CI [−0.03, 0.02], P = 0.89, BF01 > 1500; Marginal R2/Conditional R2 = = 0.003/0.19). Here, we find that the reactivation of a consolidated target memory directly affects this memory but does not seem to influence those elements acquired in a different context (peripheral memory).
Experiment 3
Materials and methods
Experiment 3 investigated whether the direct strengthening of a consolidated target memory through reactivation could spread to and benefit the acquisition of new information (peripheral memory) presented simultaneously in the same spatiotemporal context. To this end, participants performed an experiment similar to the previous ones with modifications on Day 2. On Day 1, participants only received training in target memory (paired associates) and no objects were presented. On Day 2, one group received the RI, and the other the RC, interspersed with the decision task (categorization), which served as the peripheral memory (new information). The presentation of each face during reactivation was followed by the presentation of 2 everyday objects in which participants had to answer whether they were primarily for internal or external use. The presentation of the objects here used identical parameters to those used in the decision task of Experiment 1. Finally, on Day 3, peripheral memory and target memory were assessed. A total of 90 participants participated in this study. A final number of 82 individuals (age M = 26.11, SD = 5.63; 48 women and 34 men) remained. While participants generally reported higher levels of positive affect (PANAS M = 31.15, 95% CI [29.68, 32.62]) compared to negative affect (PANAS M = 15.54, 95% CI [14.07, 17.01], F (1.178) = 219.383, P < 0.001, ηp2 = 0.56), there was ambiguous evidence for differences between the experimental groups (F (1.138) = 0.192, P = 0.662, ηp2 = 0.001, BF01 = 1.01).
Results
Target memory (paired-associates) acquisition and evaluation
Memory accuracy for the Target memory training was comparable across groups (Fig. 2, tr1 RI vs RC β = 0.71, 95% CI [0.26,1.17], P = 0.30; BF01 = 3.44, tr3 RI vs RC, β = 0.37, 95% CI [−0.73, 1.47], P = 0.51, BF01 = 4.56) but varied on Day 3 as a function on the reactivation type received (Main effects: Reactivation X2 (1) = 1.2005, P = 0.2732, Trial X2 (5) = 550.9744, P < 0.0001; Reactivation × Trial Interaction, X2 (5) = 19.6622, P = 0.0014, R2 Marginal = 0.31, R2 Conditional = 0.460). On Day 3, the RI group showed improved memory retention compared to the RC group, as indicated by the significant difference on the first testing trial (ts1 RI vs RC β = 1.70, 95% CI [0.84, 2.55], P = 0.03).
Peripheral memory (object recognition)
As in the previous experiments, we found that participants tended to classify Objects as New items (bias c: β = −0.37, 95% CI [−0.45, −0.30], P < 0.001, Marginal R2/Conditional R2 = 0.39/0.44). Also, the proportion of Old items judged as Old was higher than the Old items judged as New, revealing a reliable sensitivity (sensitivity d´: β Item Condition = 1.56, 95% CI [1.50, 1.62], P < 0.001; Reactivation β = 0.06, 95% CI [−0.08, 0.21], P = 0.38). Notably, the interaction between Reactivation and Item Condition was significant, showing that RI performed better on the peripheral memory task compared to RC (Fig. 3, MRI d´ = 1.71, 95% CI [1.63, 1.79], MRC d´ = 1.41, 95% CI [1.34, 1.49], MDifference = −0.297 [−0.38, −0.13], Cohen´s d = −0.18; Reactivation × sensitivity d´: β = −0.29, 95% CI [−0.40, −0.18], P < 0.001. Overall recognition accuracy did not differ significantly between the reactivation groups. (Fig. 3, MRI = 0.78, 95% CI [0.76, 0.79], MRC = 0.74, 95% CI [0.73, 0.75], Main effects: Reactivation X2 (1) = 1.52, P = 0.22, BF01 = 3.1, Item Condition X2 (1) = 131.41, P < 0.0001, Marginal R2/Conditional R2 = 0.19/0.35). However, we found, as in Experiment 1, that RI performed better on New items compared to RC (Reactivation × Condition Interaction X2 (1) = 5.38, P < 0.005, MRI = 0.89, 95% CI [0.87, 0.90], MRC = 0.84, 95% CI [0.82, 0.85], z = 2.08, P = 0.02). Recognition accuracy for Old items was similar in both groups (MRI = 0.66, 95% CI [0.65, 0.68], MRC = 0.63, 95% CI [0.61, 0.65], z = 0.72, P = 0.45, BF01 = 3.43). The analysis of RTs was not informative about treatment effects between groups (Reactivation × Condition Interaction β = 0.014, 95% CI [−0.009, 0.03], P = 0.23, BF01 > 1500; Reactivation β = −0.01, 95% CI [−0.07, 0.04], P = 0.60; Item Condition β = −0.004, 95% CI [−0.02, 0.01], P = 0.56); Marginal R2/Conditional R2 = 0.001/0.15). In conclusion, the present experiment suggests that reactivation of a target memory strengthens this memory and may also facilitate the acquisition of new information (peripheral memory) presented during reactivation. This would suggest a better integration of peripheral information into target memory, revealing the process of memory updating.
Experiment 4
Materials and methods
Experiment 4 served as a control for the previous experiment to examine how the shared context during the reactivation of the target memory and the presentation of new information (peripheral memory) affect the indirect strengthening effect of the peripheral memory. Here, participants performed a similar experiment to Experiment 3. On Day 1, all participants were trained on the paired associates (Target Memory). On Day 2, one group received the RI and the other the RC separated by the same autobiographical distractor task. This intervention aimed to separate the context of the reactivation of the target memory from the presentation of the decision task (peripheral memory). As in experiment 2, the peripheral object task was designed to represent a distinct context from the paired-associates task by altering the typography, font color, and background of the presented images. On Day 3, both memories were tested. A total of 51 participants participated in this study. The same exclusion criteria as experiment 1 were applied. 50 individuals (age M = 23.65, SD = 4.41; 35 women and 15 men) remained. No conclusive evidence of group differences was found in the overall pattern of affective responses (PANAS F (1.106) = 0.723, P = 0.39, ηp2 = 0.007, BF01 = 1.1), which were characterized by greater positive (M = 31.4, 95% CI [29.37, 33.50]) than negative affect (M = 18.18, 95% CI [16.12, 20.25], F (1.106) = 81.187, P < 0.001, ηp2 = 0.45).
Results
Target memory (paired-associates) acquisition and evaluation
As illustrated in Fig. 2, Day 1 memory accuracy for the Target memory training showed no significant evidence of differences between groups. At the end of training, all participants successfully reached the inclusion criterion (tr3 RI vs RC, β = −0.90, 95% CI [−2.15, 0.30], P = 0.22, BF01 = 3.31). On day 3, memory retention was modulated by the reactivation received (Main effects: Reactivation X2 (1) = 1.1929, P = 0.2747, BF01 = 3.06, Trial X2 (5) = 554.8018, P < 0.0001, Reactivation × Trial Interaction, X2 (5) = 20.01747, P = 0.001, R2 Marginal = 0.30, R2 Conditional = 0.47). Analyses revealed significantly better performance on the paired-associates task for the RI group compared to the RC group on the first testing trial of Day 3 (ts1 RI vs RC: β = 1.72, 95% CI [0.84, 2.60], P = 0.03). These findings provide consistent evidence that RI effectively reactivates and strengthens previously consolidated target memories to a higher degree than RC.
Peripheral memory (object recognition)
Data analysis indicates a similar pattern of results to Experiment 2, where the decision task was also performed separately from the acquisition-reactivation of the target memory. That is, participants generally showed a bias to classify objects as New (bias c: β = −0.38, 95% CI [−0.49, −0.27], P < 0.001, Marginal R2/Conditional R2 = 0.48/0.53) but were able to discriminate their condition (Old/New) robustly (sensitivity d´: β = 2.05, 95% CI [1.97, 2.13], P < 0.001; Reactivation β = −0.02, 95% CI [−0.23, 019], P = 0.83, BF01 = 6.3). The reactivation intervention did not affect sensitivity to classify objects as New/Old (Fig. 3, MRI d´ = 2.12, 95% CI [2.01, 2.23], MRC d´ = 1.99, 95% CI [1.88, 2.10], MDifference = −0.132 [−0.29, 0.02], Cohen´s d = −0.07; Reactivation × sensitivity d´: β = −0.13, 95% CI [−0.28, 0.26], P = 0.11, BF01 = 4.61). Regarding recognition accuracy, RI and RC performed similarly (Fig. 3, MRI = 0.83, 95% CI [0.81, 0.84], MRC = 0.81, 95% CI [0.79, 0.82], Main effects: Reactivation X2 (1) = 1.01, P = 0.31, Item Condition X2 (1) = 91.79, P < 0.0001, Marginal R2/Conditional R2 = 0.12/0.34), with no evidence for differences between Old/New Conditions (Reactivation × Condition Interaction X2 (1) = 0.04, P = 0.83; BF01 = 12.55, Old MRI = 0.91, 95% CI [0.90, 0.92], MRC = 0.90, 95% CI [0.88, 0.91], New MRI = 0.74, 95% CI [0.72, 0.76], MRC = 0.72, 95% CI [0.69, 0.74]). RT analysis also showed no significant differences between reactivation groups (Reactivation × Condition Interaction β = 0.010, 95% CI [−0.007, 0.01], P = 0.10, BF01 > 1500; Reactivation β = −0.04, 95% CI [−0.13, 0.04], P = 0.34; Item Condition β = 0.03, 95% CI [0.007, 0.05], P = 0.009; Marginal R2/Conditional R2 = 0.007/0.20). These results suggest that reactivating a target memory improves its long-term retention but does not affect new information presented separately from its reactivation-updating context (Fig. 4).
Pooled analysis across all experiments
To synthesize the findings from Experiments 1–4 within a single statistical framework and improve inference, we pooled all data and analyzed sensitivity, recognition accuracy, and its association. First, using the first trial of the target memory testing (ts1) as a measure of the strength of this memory, we found that it was positively associated with sensitivity to discriminate objects (Old/New; r = 0.21, 95% CI [0.14, 0.28], P = 0.008) and general recognition accuracy of peripheral memory (r = 0.41, 95% CI [0.20, 0.43], P < 0.001). We also found a significant correlation between sensitivity and recognition accuracy (r = 0.29, 95% CI [0.17, 0.40], P < 0.001).
Then, to show that the strengthening of peripheral memory depends on the strengthening of target memory, we target the interaction between the type of Reactivation (RI vs. RC) and the Context in which peripheral memory was acquired (same context vs. different context). For that purpose, Sensitivity and recognition accuracy were used as dependent variables, and we performed a 2 × 2 × 2 factorial ANOVA considering Reactivation (RI vs. RC), Context (same vs. different), and Day (same vs. different) as fixed factors, followed by post hoc Bonferroni t tests. Main effects results revealed that: (1) the Reactivation Intervention (RI) groups had greater sensitivity in discriminating objects than the Reactivation control (RC) groups (Mdifference = 0.18, 95% CI [0.16, 0.20], Reactivation Main effect F(1.230) = 394.667, P < 0.001, ηp2 = 0.64); (2) Peripheral memory acquired in a different context (separately as in Experiments 2 and 4) showed greater sensitivity than when this memory was acquired in the same context (Mdifference = −0.39, 95% CI [−0.40, −0.37], Context Main effect F(1.230) = 2639.088, P < 0.001, ηp2 = 0.93); (3) Peripheral memory showed greater sensitivity when it was acquired on the same day with the target memory (Experiments 1 and 2) than when it was acquired during the reactivation of the target memory (Mdifference = 0.06, 95% CI [0.04, 0.07], Day main effect F(1.230) = 57.435, P < 0.001, ηp2 = 0.20). Notably, we found evidence that the Sensitivity of peripheral memory depends on the strengthening of target memory, as indicated by the significant interaction between Reactivation × Context (F (1.230) = 280.760, P < 0.001, ηp2 = 0.56) and Reactivation × Context × Day (F (1.230) = 83.360, P < 0.001, ηp2 = 0.27). Follow-up analysis indicated that the effect of Reactivation on Sensitivity was conditioned by the acquisition/reactivation Context (RISame vs RCSame MDifference = 0.28, 95% CI [0.26, 0.29], P < 0.0001; RIDifferent vs RCDifferent MDifference = 0.02, 95% CI [0.00005, 0.04], P = 0.07). To obtain a more precise estimate of these effects, we conducted two independent 2 × 2 ANOVAs for Experiments 1–2 and Experiments 3–4. In these analyses, Reactivation and Context were included as fixed factors to examine sensitivity within each condition. The results of the variance analyses revealed that sensitivity differed based on the type of reactivation and acquisition context, regardless of whether the peripheral objects were acquired on the same day as the target memory (Experiments 1–2) or on different days (Experiments 3–4). Specifically, for Experiments 1–2, there was a significant main effect of Reactivation, F (1107) = 364.74, P < 0.001, ηp2 = 0.30, a significant main effect of Context, F (1107) = 719.05, P < 0.001, ηp2 = 0.57, and a significant Reactivation × Context interaction, F (1107) = 27.70, P < 0.001, ηp2 = 0.03. Similarly, for Experiments 3–4, there was a significant main effect of Reactivation, F (1123) = 74.90, P < 0.001, ηp2 = 0.03, a significant main effect of Context, F (1123) = 2153.53, P < 0.001, ηp2 = 0.78, and a significant Reactivation × Context interaction, F (1123) = 355.08, P < 0.001, ηp2 = 0.13. As with the sensitivity analysis, peripheral memory acquired in a different context revealed better overall object recognition performance (F (1.230) = 19.45, P < 0.01, ηp2 = 0.10, Same vs Different Context MDifference = −0.06, 95% CI [−0.07, −0.02]). In conclusion, this analysis suggests that when the target and peripheral memory context are shared, reactivation may have an indirect retroactive or proactive strengthening effect. While this pooled analysis across all experiments revealed a consistent pattern of results supporting the indirect strengthening effect of memory reactivation, it is important to interpret these findings with caution. The experiments differed in their specific methodologies, including variations in experimental manipulations, timing of peripheral memory acquisition, and reactivation contexts. These differences may introduce heterogeneity that could influence the observed effects. However, the robustness of the core finding—that reactivation of a target memory strengthens associated peripheral memories— across diverse paradigms suggests that the effect generalizes beyond specific experimental conditions.
Discussion
Memories of a conversation may include the most salient aspects of the event, such as the content of the conversation or the people involved, but also involve elements of the context in which it took place, such as the location where it happened or the time of the day or year. These latter aspects may come with subtler details, such as the aroma of the place or the temperature. When remembering such target conversation, some aspects might be inaccessible, but others may come along involuntarily and be subject to change. The present study investigated whether the direct reactivation of a consolidated episodic memory could indirectly strengthen related information. Across four experiments, we demonstrated that reactivating a consolidated Target Memory (face-name pairs) using a Reactivation Intervention (RI) strengthened not only the retention of the direct target memories but also the recognition of objects encountered in the same temporal acquisition context (Experiment 1). This indirect strengthening effect was contingent upon a shared context, as it was absent when the objects were learned after a temporal delay and an intervening autobiographical memory task (Experiment 2). Furthermore, presenting new objects concurrently with the reactivation of the target memory also resulted in improved recognition of these new objects (Experiment 3), but only when they shared a direct contextual link with the reactivated memories (Experiment 4). These findings suggest that the reactivation of a consolidated memory can trigger the strengthening of associated information, highlighting the integrative nature of episodic memory and the potential for leveraging temporal contextual reinstatement to improve learning and memory. Importantly, we found that not all reactivation leads to such improved memory retention. Thus, based on previous experiments25, we believe that the RI may include a prediction error component (the incongruence between what was anticipated and what occurred in the task) that triggers the reactivation–reconsolidation process. While traditional accounts of prediction error emphasize unexpected deviations from anticipated outcomes, the reconsolidation literature often defines prediction error more flexibly, encompassing structural mismatches between encoding and reactivation conditions. In our study, the RI introduced an incomplete cue presentation and an unexpected interruption, which initially may have generated a moderate prediction error. However, as the trials progressed, participants likely adapted to this structure, reducing the element of surprise. This suggests that the observed memory effects may not solely rely on prediction error but also on retrieval-related processes. An alternative interpretation is that RI functioned similarly to retrieval practice, strengthening target memories through reactivation rather than through reconsolidation-driven labilization. In contrast, the Reactivation Control (RC) condition, which lacked the incomplete cue presentation, may have functioned more like a restudy condition, leading to less pronounced memory benefits. Crucially, this indirect strengthening manifested as an increased sensitivity in discriminating between old and new objects rather than a general increase in accuracy or a shift in response bias. This suggests that reactivating a target memory improves the discriminability of related contextual information, making it easier to distinguish from new. Overall, these findings support the notion that episodic memories are stored and retrieved in an associative manner62,63, consistent with theories proposing that episodic memory relies on relational representations, wherein individual items are interconnected through their shared context.
Another aspect of context effects is their general role in peripheral memory acquisition (decision task) and subsequent performance on Day 3. Independently of the reactivation received (RI vs RC), we found that peripheral memory showed a greater sensitivity and accuracy when acquired uninterruptedly by target memory and in a separate temporal context (Experiments 2 and 4). It could be considered that the interleaved presentation of objects during the acquisition (Experiment 1) or reactivation (Experiment 3) of face-name pairs impoverishes their encoding by attentional mechanisms or competition for shared neurocognitive resources64. In this sense, it is possible that target memory is prioritized, given that it is the instruction that the participants received and that it generates proactive interference on the incidental acquisition of peripheral memory. A recent report by Kozak et al.65 provides similar evidence: they found that when participants were instructed to learn a list of words interspersed with scene images and then instructed to retain the words, they showed poor recognition of the images in a final evaluation. However, if after the same training, participants were asked to forget the words, they achieved much better performance in recognizing the images, despite not receiving instructions to learn or remember the images in either case. These results support a competition between memory for content and context, suggesting that these two memories rely on common neurocognitive resources. A complementary interpretation is provided by the work of Davids et al.66, who highlighted that frequent alternation between retrieval and encoding processes can hinder the learning of new material due to interference between distinct cognitive mechanisms. In Experiments 1 and 3, the interleaved presentation of objects during the acquisition and reactivation phases likely introduced such interference, competing for attentional and neurocognitive resources and thus impairing the encoding of peripheral objects. In contrast, the separation of these phases in Experiments 2 and 4 may have minimized this interference, facilitating improved encoding and retrieval of peripheral memories.
Previous studies of reactivation interventions in consolidated memories tried to elucidate if the effects were specific to the directly reactivated items. They found that the treatment only affected the reactivated items and left the non-reactivated aspects unchanged67, concluding that reconsolidation can only modulate the directly reactivated elements. The current study extends our understanding of memory consolidation and reconsolidation by demonstrating that these processes can impact not only the directly reactivated memory and related contextual information. While previous research has shown that reactivating a consolidated memory can strengthen, update with new information, or even weaken the original trace8,9, our results suggest that reconsolidation can also lead to the strengthening of associated information that is not directly cued. In addition, our findings (Experiments 3 and 4) suggest that reactivation not only strengthens the target memory but may also facilitate the integration of new information (peripheral objects) presented during reactivation into the original memory trace. This is consistent with the concept of memory updating within the framework of reconsolidation. When reactivated, consolidated memories enter a labile state, during which new, temporally or contextually linked information can be incorporated into the existing memory representation before re-stabilization. The improved recognition of objects observed in these experiments may reflect their incorporation into the associative structure of the reactivated face-name memory, effectively updating it to include peripheral details encountered during reactivation. These findings raise intriguing questions about the scope and mechanisms of episodic memory reconsolidation, suggesting a more distributed and context-dependent process44. Similarly, a report by Hupbach et al.68 found that spatial context is a necessary condition for the occurrence of reconsolidation. They showed the existence of asymmetric updating (intrusions) in episodic memories only when the context of acquisition and reactivation were the same but not when they changed.
While our findings emphasize reconsolidation and memory updating as key mechanisms, alternative explanations, and mechanisms also offer valuable insights into the observed effects. For example, the RI may have enhanced attention to the task. Participants might have anticipated a memory test, as on Day 1, or entered a more active cognitive observing the reminder, leading to deeper encoding of peripheral stimuli in Experiments 3 and 4. Although reaction time analyses showed no significant differences between RI and RC groups during the task, subtle attentional differences cannot be ruled out. Additionally, RI may have acted as a retrieval practice, enhancing target memory retention while indirectly facilitating peripheral memories through retrieval-induced facilitation (RIFA)14. Specifically, RIFA may explain the enhanced sensitivity and accuracy for peripheral memories observed in the RI groups, as retrieving the target items could have activated their episodic context and indirectly strengthened contextually associated memories55. The RI might have enhanced the encoding of new peripheral items (Experiments 3 and 4) by inducing a heightened cognitive state, aligning with the forward testing effect, where prior retrieval facilitates the learning of subsequent information69. Moreover, spreading activation models propose that memory retrieval triggers the activation of semantically or contextually related memories through associative networks14. Reactivating a target memory during the RI could have activated related peripheral memories via these associative pathways. This activation may have increased the accessibility and subsequent retention of peripheral items, particularly those closely linked to the target memory by shared spatiotemporal features. Finally, our findings may also be explained by the context-item associations framework, which emphasizes the binding of memories to their acquisition context70. In Experiments 1 and 3, the shared context between target and peripheral memories during acquisition and reactivation likely played a significant role in the observed indirect strengthening effects. The RI may have reinstated the original context, facilitating the retrieval of both target and peripheral memories. This mechanism is particularly relevant in explaining why the strengthening effect diminished in Experiments 2 and 4, where the context of peripheral memory acquisition was deliberately separated from the target memory55. Another complementary interpretation of the indirect strengthening effect corresponds to the Behavioral Tagging hypothesis, which serves as the behavioral analog to the synaptic tagging and capture hypothesis36,71. The findings indicate that direct reactivation of target memories not only enhances the strength of a strong memory (target memory) but also facilitates the retention of weak, non-reactivated related information (peripheral memory). In this sense, the reactivation process may act as a behavioral marker, tagging the associated information for subsequent encoding and (re)consolidation. The observed strengthening in memory retention could imply that the reactivation of neural pathways during memory retrieval facilitates the system to capture new contextual cues and related information, effectively integrating them into the pre-existing memory framework. Thus, the study highlights the dynamic nature of memory processes, where reactivation not only stabilizes existing memories but also enables the incorporation of new information (updating), reinforcing and complementing the theoretical framework established by behavioral tagging and reconsolidation.
The indirect strengthening through reactivation may offer potential benefits for both educational strategies and memory rehabilitation. In educational settings, incorporating targeted reactivation exercises within a specific learning context could enhance the retention of not only the directly reviewed material but also related information presented within the same temporal or semantic context. This approach could be particularly effective for individuals with memory impairments, as it leverages the power of contextual reinstatement. By revisiting key concepts or events and then encouraging the retrieval of associated details, educators and therapists can potentially strengthen the connections between memories and improve overall retention.
Limitations
One limitation of this study is that while we observed indirect strengthening of contextually bound peripheral memories following target reactivation, the precise neural mechanisms underlying this effect remain to be fully elucidated. Future research employing neuroimaging techniques could provide further insights into the brain regions and processes involved. Additionally, the current study focused on a specific type of associative memory (face-name pairs with peripheral objects) and a single reactivation protocol. Further investigation is needed to determine the generalizability of these findings to other types of memories, prediction errors, and different reactivation manipulations. Finally, while the sample size was adequate for detecting the main effects, larger and more diverse samples could enhance the robustness and generalizability of the observed indirect strengthening effect.
Conclusion
Our results demonstrate that reactivation strengthens the target memory, as expected, but also extends to improve the retention of non-reactivated memories that share a common context. Specifically, experiments showed that robust reactivation enhanced the accuracy of face-name pairs and improved the recognition of associated peripheral objects, supporting the idea that memory strengthening through reactivation can spread across related memories. This indirect strengthening effect appeared to be influenced by the context in which memories were acquired and reactivated, with stronger effects observed when both types of memories were acquired in the same context.
Data availability
All data for these experiments have been made publicly available in an anonymized form at the OSF: https://osf.io/4fu9q/files/osfstorage.
Code availability
The code for the analysis is found at https://osf.io/qfexs.
References
Dudai, Y. The neurobiology of consolidations, or, how stable is the engram? Annu. Rev. Psychol. 55, 51–86 (2004).
Hu, X., Cheng, L. Y., Chiu, M. H. & Paller, K. A. Promoting memory consolidation during sleep: a meta-analysis of targeted memory reactivation. Psychol. Bull. 146, 218 (2020).
Robertson, E. M. & Genzel, L. Memories replayed: reactivating past successes and new dilemmas. Philos. Trans. R. Soc. B 375, 20190226 (2020).
Dudai, Y. The restless engram: consolidations never end. Annu. Rev. Neurosci. 35, 227–247 (2012).
Paller, K. A. Recurring memory reactivation: the offline component of learning. Neuropsychologia 196, 108840 (2024).
Agren, T. Human reconsolidation: a reactivation and update. Brain Res. Bull. 105, 70–82 (2014).
Wichert, S., Wolf, O. T. & Schwabe, L. Updating of episodic memories depends on the strength of new learning after memory reactivation. Behav. Neurosci. 127, 331–338 (2013).
Roediger, H. & Karpicke, J. Test-enhanced learning taking memory tests improves long-term retention. Psychol. Sci. 17, 249–255 (2006).
McDermott, K. B. The persistence of false memories in list recall. J. Mem. Lang. 35, 212–230 (1996).
Roediger, H. L. III. & Abel, M. The double-edged sword of memory retrieval. Nat. Rev. Psychol. 1, 708–720 (2022).
Karpicke, J. D., Lehman, M. & Aue, W. R. Retrieval-based learning: An episodic context account. In The Psychology of Learning and Motivation (ed. Ross, B. H.) 237–284 (Elsevier Academic Press, 2014).
Alvares, L. D. O. et al. Reactivation enables memory updating, precision-keeping and strengthening: exploring the possible biological roles of reconsolidation. Neuroscience 244, 42–48 (2013).
Chan, J. C. Long-term effects of testing on the recall of nontested materials. Memory 18, 49–57 (2010).
Rowland, C. A. & DeLosh, E. L. Benefits of testing for nontested information: retrieval-induced facilitation of episodically bound material. Psychon. Bull. Rev. 21, 1516–1523 (2014).
Chan, J. C., McDermott, K. B. & Roediger, H. L. III. Retrieval-induced facilitation: initially nontested material can benefit from prior testing of related material. J. Exp. Psychol. Gen. 135, 553 (2006).
Cho, K. W., Neely, J. H., Crocco, S. & Vitrano, D. Testing enhances both encoding and retrieval for both tested and untested items. Q. J. Exp. Psychol. 70, 1211–1235 (2017).
Bäuml, K.-H. T. Context retrieval as a critical component in selective memory retrieval. Curr. Dir. Psychol. Sci. 28, 177–182 (2019).
Jonker, T. R., Dimsdale-Zucker, H., Ritchey, M., Clarke, A. & Ranganath, C. Neural reactivation in parietal cortex enhances memory for episodically linked information. Proc. Natl. Acad. Sci. USA 115, 11084–11089 (2018).
Butler, A. C. Repeated testing produces superior transfer of learning relative to repeated studying. J. Exp. Psychol. Learn. Mem. Cogn. 36, 1118–1133 (2010).
Bäuml, K.-H. T. & Samenieh, A. The two faces of memory retrieval. Psychol. Sci. 21, 793–795 (2010).
Roediger, H. L. Recall as a self-limiting process. Mem. Cogn. 6, 54–63 (1978).
Chan, J. C. When does retrieval induce forgetting and when does it induce facilitation? Implications for retrieval inhibition, testing effect, and text processing. J. Mem. Lang. 61, 153–170 (2009).
Roediger, H. L. & Karpicke, J. D. The power of testing memory: basic research and implications for educational practice. Perspect. Psychol. Sci. 1, 181–210 (2006).
Forcato, C., Rodríguez, M. L. C. & Pedreira, M. E. Repeated labilization-reconsolidation processes strengthen declarative memory in humans. PLoS ONE 6, e23305 (2011).
Fernández, R. S. et al. Improvement of episodic memory retention by a memory reactivation intervention across the lifespan: from younger adults to amnesic patients. Transl. Psychiatry 12, 144 (2022).
Fernández, R. S., Bavassi, L., Kaczer, L., Forcato, C. & Pedreira, M. E. Interference conditions of the reconsolidation process in humans: the role of valence and different memory systems. Front. Hum. Neurosci. 10, 641 (2016).
Forcato, C., Fernandez, R. S. & Pedreira, M. E. The role and dynamic of strengthening in the reconsolidation process in a human declarative memory: what decides the fate of recent and older memories? PLoS ONE 8, e61688 (2013).
Liu, J. et al. An unconditioned stimulus retrieval extinction procedure to prevent the return of fear memory. Biol. Psychiatry 76, 895–901 (2014).
Debiec, J., Diaz-Mataix, L., Bush, D. E., Doyère, V. & LeDoux, J. E. The selectivity of aversive memory reconsolidation and extinction processes depends on the initial encoding of the Pavlovian association. Learn. Mem. 20, 695–699 (2013).
Fernández, R. S., Boccia, M. M. & Pedreira, M. E. The fate of memory: reconsolidation and the case of prediction error. Neurosci. Biobehav. Rev. 68, 423–441 (2016).
Pedreira, M. E., Pérez-Cuesta, L. M. & Maldonado, H. Mismatch between what is expected and what actually occurs triggers memory reconsolidation or extinction. Learn. Mem. 11, 579–585 (2004).
Chalkia, A., Van Oudenhove, L. & Beckers, T. Preventing the return of fear in humans using reconsolidation update mechanisms: a verification report of Schiller et al. (2010). Cortex 129, 510–525 (2020).
Schiller, D. & Phelps, E. A. Does reconsolidation occur in humans? Front. Behav. Neurosci. 5, 24 (2011).
Fernández, R. S., Pedreira, M. E. & Boccia, M. M. Does reconsolidation occur in natural settings? Memory reconsolidation and anxiety disorders. Clin. Psychol. Rev. 57, 45–58 (2017).
Scully, I. D., Napper, L. E. & Hupbach, A. Does reactivation trigger episodic memory change? A meta-analysis. Neurobiol. Learn Mem. 142, 99–107 (2017).
Moncada, D., Ballarini, F. & Viola, H. Behavioral tagging: a translation of the synaptic tagging and capture hypothesis. Neural Plast. 2015, 650780 (2015).
Allen, T. A. & Fortin, N. J. The evolution of episodic memory. Proc. Natl. Acad. Sci. USA 110, 10379–10386 (2013).
Tulving, E. Elements of Episodic Memory (Oxford University Press, 1983).
Rolls, E. T. The mechanisms for pattern completion and pattern separation in the hippocampus. Front. Syst. Neurosci. 7, 74 (2013).
Josselyn, S. A. & Frankland, P. W. Memory allocation: mechanisms and function. Annu. Rev. Neurosci. 41, 389–413 (2018).
Josselyn, S. A. & Tonegawa, S. Memory engrams: recalling the past and imagining the future. Science 367, eaaw4325 (2020).
Ranganath, C. Binding items and contexts: the cognitive neuroscience of episodic memory. Curr. Dir. Psychol. Sci. 19, 131–137 (2010).
Horner, A. J., Bisby, J. A., Bush, D., Lin, W.-J. & Burgess, N. Evidence for holistic episodic recollection via hippocampal pattern completion. Nat. Commun. 6, 7462 (2015).
Yonelinas, A. P., Ranganath, C., Ekstrom, A. D. & Wiltgen, B. J. A contextual binding theory of episodic memory: systems consolidation reconsidered. Nat. Rev. Neurosci. 20, 364–375 (2019).
Eichenbaum, H. On the integration of space, time, and memory. Neuron 95, 1007–1018 (2017).
Andermane, N., Zhai, C., Henderson, L. & Horner, A. J. The holistic forgetting of events and the (sometimes) fragmented forgetting of objects. Cognition 255, 106017 (2025).
Anwyl-Irvine, A. L., Massonnié, J., Flitton, A., Kirkham, N. & Evershed, J. K. Gorilla in our midst: An online behavioral experiment builder. Behav. Res. 52, 388–407 (2020).
Medrano, L. A. et al. Adaptación de la Escala de Afecto Positivo y Negativo (PANAS) para la población de Estudiantes Universitarios de Córdoba. Anuario de. Investigaciones de. la Facultad de. Psicol.ía 2, 22–36 (2015).
Fernández, R. S. et al. Psychological distress and mental health trajectories during the COVID-19 pandemic in Argentina: a longitudinal study. Sci. Rep. 12, 5632 (2022).
Ebner, N. C., Riediger, M. & Lindenberger, U. FACES—a database of facial expressions in young, middle-aged, and older women and men: development and validation. Behav. Res. Methods 42, 351–362 (2010).
Kirwan, C. B. & Stark, C. E. L. Overcoming interference: an fMRI investigation of pattern separation in the medial temporal lobe. Learn. Mem. 14, 625–633 (2007).
Watson, D., Clark, L. A. & Tellegen, A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Personal. Soc. Psychol. 54, 1063–1070 (1988).
Forcato, C., Fernandez, R. S. & Pedreira, M. E. Strengthening a consolidated memory: the key role of the reconsolidation process. J. Physiol. 108, 323–333 (2014).
Whitmore, N. W., Harris, J. C., Kovach, T. & Paller, K. A. Improving memory via automated targeted memory reactivation during sleep. J. Sleep. Res. 31, e13731 (2022).
Pan, S. C. & Rickard, T. C. Transfer of test-enhanced learning: meta-analytic review and synthesis. Psychol. Bull. 144, 710–756 (2018).
Forcato, C., Argibay, P. F., Pedreira, M. E. & Maldonado, H. Human reconsolidation does not always occur when a memory is retrieved: the relevance of the reminder structure. Neurobiol. Learn. Mem. 91, 50–57 (2009).
Fernández, R. S., Bavassi, L., Forcato, C. & Pedreira, M. E. The dynamic nature of the reconsolidation process and its boundary conditions: evidence based on human tests. Neurobiol. Learn. Mem. 130, 202–212 (2016).
R Core Team. R: a language and environment for statistical computing. (R Foundation for Statistical Computing, Vienna, Austria, 2021).
Singmann, H. & Kellen, D. An introduction to mixed models for experimental psychology. In New Methods in Cognitive Psychology (eds Spieler, D. H. & Schumacher, E.) 4–31 (Psychology Press, 2019).
Brady, T. F., Robinson, M. M., Williams, J. R. & Wixted, J. T. Measuring memory is harder than you think: how to avoid problematic measurement practices in memory research. Psychon. Bull. Rev. 30, 421–449 (2023).
Smith, S. M. & Vela, E. Environmental context-dependent memory: a review and meta-analysis. Psychon. Bull. Rev. 8, 203–220 (2001).
Tulving, E. Episodic memory: from mind to brain. Annu. Rev. Psychol. 53, 1–25 (2002).
Cohen, N. J. & Eichenbaum, H. B. Memory, Amnesia, and Hippocampal Function (MIT Press, 1989).
Marois, R. & Ivanoff, J. Capacity limits of information processing in the brain. Trends Cogn. Sci. 9, 296–305 (2005).
Kozak, S., Herz, N., Bar-Haim, Y. & Censor, N. Indirect modulation of human visual memory. Sci. Rep. 11, 7274 (2021).
Davis, S. D., Chan, J. C. K. & Wilford, M. M. The dark side of interpolated testing: frequent switching between retrieval and encoding impairs new learning. J. Appl. Res. Mem. Cogn. 6, 434–441 (2017).
Dębiec, J., Doyère, V., Nader, K. & LeDoux, J. E. Directly reactivated, but not indirectly reactivated, memories undergo reconsolidation in the amygdala. Proc. Natl. Acad. Sci. USA 103, 3428–3433 (2006).
Hupbach, A., Hardt, O., Gomez, R. & Nadel, L. The dynamics of memory: context-dependent updating. Learn. Mem. 15, 574–579 (2008).
Yang, C., Potts, R. & Shanks, D. R. Enhancing learning and retrieval of new information: a review of the forward testing effect. NPJ Sci. Learn. 3, 1–9 (2018).
Liu, Z., Johansson, M., Johansson, R. & Bramão, I. The effects of episodic context on memory integration. Sci. Rep. 14, 30159 (2024).
Moncada, D. & Viola, H. Induction of long-term memory by exposure to novelty requires protein synthesis: evidence for a behavioral tagging. J. Neurosci. 27, 7476–7481 (2007).
Acknowledgements
This work was supported by Agencia Nacional de Promoción Científica y Tecnológica PICT 2020-00956. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors would like to thank all participants, Damian Testori, Pablo Yonamine and Sebastian Eiguren for critical comments.
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Psychology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Jesse Rissman and Marike Schiffer. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Beron, J.C., Bavassi, L., Pedreira, M.E. et al. Evidence for indirect strengthening through reactivation of contextually bound memories. Commun Psychol 3, 68 (2025). https://doi.org/10.1038/s44271-025-00250-5
Received: 03 October 2024
Accepted: 10 April 2025
Published: 23 April 2025
DOI: https://doi.org/10.1038/s44271-025-00250-5
.png)




![Natural Language Processing of army insider threat hub data [pdf]](https://news.najib.digital/site/assets/img/broken.gif)

