Abstract
Sleep influences health and is affected by dehydration. Therefore, our aim was to assess the effects of mild dehydration and subsequent rehydration on sleep time and sleep quality using subjective measures. On 4 consecutive mornings, 18 males (mean ± SD; age, 23 ± 4 years; height, 175.8 ± 5.7 cm; weight, 80.1 ± 9.7 kg) reported to the laboratory with different hydration states (day 1 baseline; day 2 euhydrated; day 3 dehydrated; day 4 following 24-h ad libitum drinking, AD). First-morning urine specific gravity, urine color, urine osmolality, and body mass loss were used to assess hydration status. Sleep was reported using the Karolinska sleep diary, which measures sleep duration, sleep quality, perceived ease of falling asleep, sleep calmness, number of dreams, and perceived ease of falling asleep measured by a 5-point Likert scale. The visual analog scale measured fatigue before sleeping with each hydration state. Repeated measure analysis of variance with Bonferroni post hoc comparisons was used determining effects of hydration status on sleep. Sleep duration was significantly greater when dehydrated (7.5 ± 1.3 h) compared to baseline (6.4 ± 1.4 h, p = 0.001), euhydrated (6.7 ± 1.4 h, p = 0.006), and AD (6.9 ± 1.0 h, p = 0.024). More difficulty sleeping was also reported when dehydration (3 ± 1) than AD (4 ± 1, p = 0.001). Other measures were not significantly different (p > 0.05). Fatigue was significantly greater during dehydration before sleeping (21.93 ± 13.07) compared to euhydration before sleeping (30.23 ± 14.03, p = 0.049). Our results indicate mild dehydration increased sleep duration because of increased fatigue and decreased perceived ease of falling asleep. This suggests that maintaining euhydration may assist with perceived ease of falling asleep and feelings of fatigue.
Similar content being viewed by others
Introduction
Hypohydration has been shown to decrease physical performance [1], cognitive function [2], and overall quality of life. Losing more than 1–2% in body weight can be classified as a mildly dehydrated state [3]. According to Armstrong et al., 25–33% of the adult population are mildly dehydrated in the USA and Europe [4,5,6,7]. Mild dehydration can be achieved by consuming less than 1.5 L of water in a day [4,5,6,7] or when fluid balance is negative, which can be induced through exercise or a low water intake.
Similar to hydration, sleep can heavily influence physical and cognitive performance as well as alter mood and motor function [8]. The Centers for Disease Control and Prevention recommends that the average adult sleep at least 7 h per night; however, at least one-third of the adult population does not get the recommended amount of sleep [9]. Sleep is an essential part of human health, and sleep deprivation has been linked to depression [10], cardiovascular disease [11], and Alzheimer’s [12].
Previous studies have investigated the effects of dehydration on sleep. Mildly dehydrated women were not as alert, increased feelings of sleepiness, and experienced confusion following fluid intake restriction overnight [13]. Another study induced mild dehydration by fluid restriction found that there were no significant interactions between dehydration and sleep quality and quantity [14]. Aside from mild dehydration through water restriction, during Ramadan, which involves food and water fasting, individuals become dehydrated [15], and experience increased incidence of sleep disturbances and decreased sleep time [15,16,17].
Currently, the effects of dehydration on sleep are inconclusive. The effects of rehydration after dehydration and the dynamic effects of varying hydration states over days on sleep quality is not well understood despite that this may be the real-life daily experience of individuals with effects of a major health and performance factor, sleep. Thus, the purpose of this study was to quantify the effects of varying hydration states over multiple days, including dehydration and rehydration protocols to achieve hypohydrated and euhydrated states on measures of sleep health including sleep duration, sleep quality, perceived ease of falling asleep, sleep calmness, number of dreams, and perceived ease of falling asleep. The current study will aid in understanding how sleep measurements are impacted by different hydration states.
Methods
Participants
Eighteen participants were recruited (mean ± standard deviation [SD]; age, 23 ± 4 years; height, 175.8 ± 5.7 cm; weight, 80.1 ± 9.7 kg; 26.0 ± 3.5 BMI) and briefed on the study and the procedures of the experiment, also providing written and informed consent to participate in the study. Each participant was given a medical history questionnaire that excluded those with kidney disease or chronic illnesses that altered fluid-electrolyte balance, the use of medications affecting kidney function (e.g., diuretics or antihypertensives), tobacco use, or conditions such as diabetes. This questionnaire and exclusion criteria were verified by a physician. This study conducted convenience sampling recruited from the university campus in males between 21 and 35 years. Previous research in this community and population has demonstrated that the selected age ranges were compliant with experimental instruction. Sample size calculation was determined by body mass loss, an indicator of each participants’ dehydration level. Previous research conducted in which the standard deviation of body mass loss was 0.8%, the between subject variability day to day was around 50%. Assuming a minimum detectable body mass loss of 1.5%, an effect size of 0.2, and a type I error probability of 5%, the minimum sample size required to detect a significant difference (p < 0.05) was calculated as n = 12. The final sample size of this study (n = 18).
The research conducted was part of a larger study which investigated other research questions, resulted in multiple manuscripts, and was approved by the university Institutional Review Board [18,19,20]. The following data, tables, and figures are original to this manuscript to avoid duplication of data.
Procedures
At the beginning of the study and each day, participants were told to abstain from caffeine, alcohol, and exercise. Day 1 was a baseline day (baseline) in which participants came to the laboratory and fasted upon awakening between 7:00 am and 8:00 am with their normal hydration status (Fig. 1). Baseline measures aimed to assess habitual fluid intake behaviors and their corresponding hydration states. At the laboratory, sleep was assessed with the Karolinska Sleep Diary (KSD), a self-reported sleep questionnaire, that has a strong correlation with polysomnography [21]. The KSD has 11 questions that are used to measure multiple aspects of perceived sleep quality, sleep quantity, ease of falling asleep, sleep calmness, number of dreams, and ease of falling asleep [21]. Each question was presented on a 5-point Likert scale with a score of “1” associated with inadequate sleep measures and “5” associated with positive sleep measures. Additionally, subjects reported fatigue on the visual analog scales (VAS) during the evening before sleeping. The VAS consisted of a statement and a 100-mm straight line marked at both ends using opposite adjectives. One end of the line represented a low rating (fatigue), and the other end would have a high rating (energized). Fatigue was presented on a page-sized at 5 cm × 20 cm.
Then, first-morning spot urine samples were taken to assess urine specific gravity (USG) with a refractometer (model 300CL, Atago Co, Tokyo, Japan), urine color utilizing a validated chart [22], and body mass with minimal clothes (undergarments only) was measured (model DS44L, Ohaus Inc, Florham Park, NJ). Additionally, blood was drawn from an antecubital vein to assess plasma osmolality.
Following baseline measurements, participants left the lab, continued normal activities (excluding physical activity outside the lab, caffeine intake, and alcohol consumption). Participants were instructed to perform 24-h urine collection, fill out and return the KSD, and arrive at the laboratory between 0700 and 0800. Also, participants were instructed to consume 500 ml of water in the evening to arrive at the laboratory on the morning of day 2 in the euhydrated state (EUH). Once participants arrived in the morning, the 24-h urine sample was collected and the KSD, first-morning spot urine sample, body mass, and blood draw were performed. The 24-h urine was used to assess urine osmolality using the freezing-point depression method (model OsmoPRO, Advanced Instruments, Inc., Norwood, MA). Following the morning visit of day 2, participants did not consume fluids and ate dry foods into the morning of day 3 to arrive at the laboratory in a dehydrated state (DEH). On the morning of day 3, participants arrived at the lab and completed the same measurements on day 2 along with the 24-h urine sample and KSD. Following the morning of day 3, subjects were able to drink and eat without restrictions. Participants came on the morning of day 4 and the same assessments were performed to determine their hydration status following ad libitum drinking (AD).
Percent body mass loss was calculated based on body mass at EUH as a baseline value for each visit. The interpretation of hydration state was made at EUH after instructions for hydration were provided given the BASELINE (i.e., first visit to the laboratory) was not reflective of a starting point euhydrated state given many individuals may not maintain hydration state habitually. Hydration measurements, the KSD, and the VAS for fatigue were analyzed with repeated measures ANOVA with Bonferroni post hoc comparison. The assumption of local sphericity was examined with Mauchly’s test of sphericity. Data were reported as mean ± SD. All statistical analyses were performed using SPSS software (v.25. IBM Corporation, Armonk, NY). Significance was set a priori at p ≤ 0.05.
Results
Table 1 shows hydration measurements (percent body mass loss, first-morning USG and first-morning urine color, and 24-h urine osmolality) that the intervention was successful (i.e., euhydrated and dehydrated).
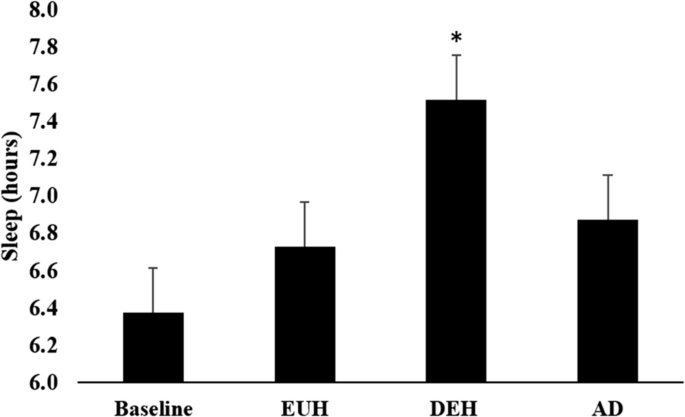
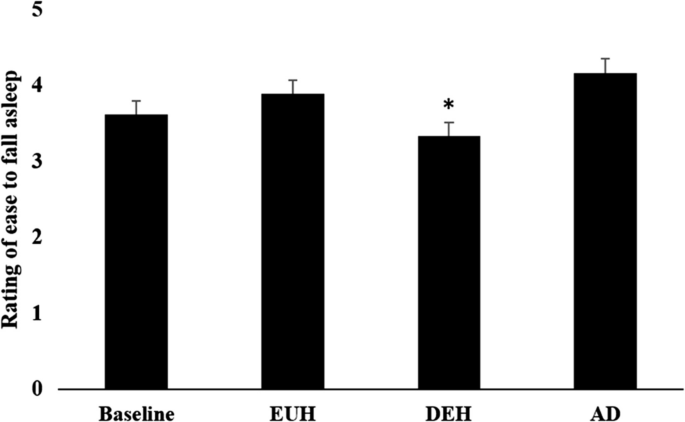
Table 2 represents KSD values and the difference between the days. In DEH (7.5 ± 1.3 h), subjects slept significantly longer than BASE (6.4 ± 1.4 h, p = 0.001), EUH (6.7 ± 1.4 h, p = 0.006), and AD (6.9 ± 1.0 h, p = 0.024) (Fig. 2). Participants subjectively reported more difficulty falling asleep, DEH (3 ± 1) from AD (4 ± 1) (p < 0.05) (Fig. 3). Although nonsignificant, EUH (4 ± 1) was close to significance different from DEH (3 ± 1) (p = 0.056). Other questionnaire items such as “How long did it take you to fall asleep?”, “How did you sleep?”, “How refreshed did you feel after awakening?”, How calmly did you sleep?”, “Did you sleep through the allotted time?”, “Number of awakenings?”, “How easy was waking up?”, and “How much did you dream?” were not significant between different hydration states (p > 0.05).
Hours of sleep were described using mean ± standard error (SE). Hours of sleep were documented on day 1 (baseline), day 2 morning (EUH), day 3 morning (DEH), and day 4 morning (AD). All data was collected on weekdays. * Significance comparing DEH versus BASE (p = 0.001), DEH versus EUH (p = 0.006), and DEH versus AD (p = 0.024)
The rating of ease of falling asleep was described using mean ± SD. Subjects rated on a scale of 1 to 5 how easy falling asleep was on day 1 (baseline), day 2 morning (EUH), day 3 morning (DEH), and day 4 morning (AD). All data was collected on weekdays. *Significance comparing DEH versus BASE, DEH versus EUH, and DEH versus AD (p < .05)
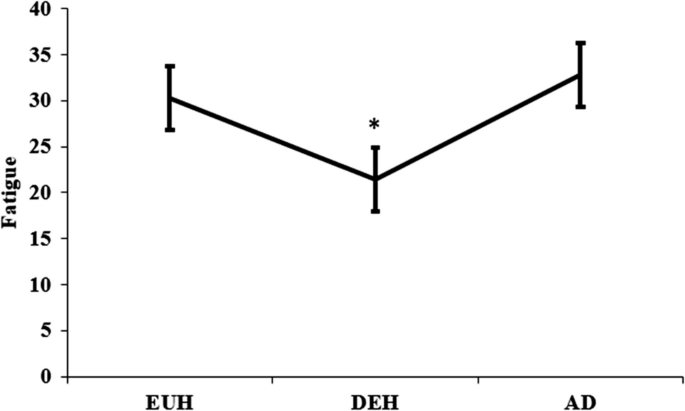
Figure 4 represents VAS evening fatigue values between each day. In DEH (21.93 ± 13.07), subjects were significantly more fatigued than in EUH (30.23 ± 14.03, p = 0.049). There were no significant differences between AD (32.82 ± 15.42) and DEH (p = 0.64) or AD and EUH (p = 0.61). The BASE evening was not measured.
The rating of fatigue was described using mean ± SE. Subjects rated fatigue on a 100 mm straight line where 0 was associated with feelings of fatigue while 100 was associated with feeling energized. This was measured on day 2 evening was (EUH), day 3 evening (DEH), and day 4 evening (AD). Day 1 (BASE) was not measured, and all data was collected on weekdays. * Difference between DEH versus EUH (p < .05)
Discussion
The aim of this study was to examine varying hydration states over multiple days and effects on sleep measures. According to the KSD, during each hydration state, the subjects did not experience any statistically significant differences in sleep quality, number of dreams, or number of awakenings. Therefore, the main findings of this study are that sleep time significantly increased during mild dehydration (DEH) than on all other days (baseline, EUH, AD), subjects reported falling asleep easier on AD compared to DEH, and evening fatigue was greater in DEH than in EUH.
Inducing mild dehydration through fluid restriction increased fatigue perception in men [23] and women [13, 24]. This can be explained by the dopaminergic and noradrenergic systems located in the brainstem responsible for attention, motivation, and fatigue, which is influenced by dehydration [25]. The findings of this study suggest that with increased fatigue experienced, sleep time increased in this study. Unlike the relationship between fatigue and hydration, scientific literature still lacks data that examines the relationship between the perceived difficulty of falling asleep—to our knowledge, this is the first study that found significance in this item of the KSD regarding dehydration.
A previous study used polysomnography, the gold standard of sleep measurement, and found no significance in mild dehydration on sleep latency and sleep duration [14]. Similar to our findings, there was no significance in sleep latency; however, we found that sleep duration increased while mildly dehydrated. Although the findings were done through the KSD, the questionnaire has been validated in previous studies with polysomnography [26, 27]. The conflicting data on sleep duration could be attributed to not assessing the percent body mass loss across baseline and dehydration conditions. Analyzing percent body mass loss helps demonstrate the degree to which individuals are dehydrated. As a result, the absence of the data demonstrates an unknown dehydration status. The level of dehydration may impact sleep measurements such as sleep duration and quality; however, conclusions cannot be definitely drawn and could possibly be the difference in the results.
In contrast, fasting for Ramadan has been found to dehydrate individuals [15], increase sleep latency [16, 17], and decrease sleep duration by \(\sim\) 1 h [17]. As opposed to dehydration through fluid restriction, Ramadan requires individuals to refrain from consuming both food and water. Although the link between dehydration and sleep is significant [28], there is also a relationship between food and sleep [29]. Food restriction during Ramadan results in hunger [30]. Hunger is primarily influenced by ghrelin, an endocrine hormone, and increases in ghrelin have also been associated with sleep loss [29]. Therefore, the relationship between sleep loss and dehydration during Ramadan is unclear.
A limitation of this study was that the participants recruited were male college-aged individuals. The protocol required participants to report to the laboratory between 7:00 am and 8:00 am, potentially influencing habitual living patterns, therefore, increasing sleep time during DEH. Despite increased sleep time, fatigue increases with DEH were consistent with previous literature. Furthermore, female subjects were not recruited and need to be tested.
A major limitation in sleep studies is the preliminary pursuit of testing effects of states such as hypohydrated or euhydrated state on measures of sleep that are self-report and include subject measures of sleep quality that cannot be collected blinded to an obvious physical state induced by behavioral interventions (e.g., not drinking fluids over the course of multiple hours to induce dehydration). This study used self-report and subjective measures of sleep gathered through a validated, but still limited in interpretation, KSD tool. Our data provide preliminary evidence to pursue more invasive and objective measures of sleep.
Conclusion
The findings in this study suggest that euhydration followed by dehydration increased the perception of difficulty falling asleep and increased sleep duration. These findings emphasize that dehydration may play an impactful role in sleep. Populations who are prone to mild dehydration such as older adults [31], children [32, 33], or athletes who exercise in the heat [34] may be able to benefit from improved fatigue and improved feelings of falling asleep faster. Aside from these populations, this study suggests that athletes may benefit psychologically by falling asleep faster and provides support recommendations for athletes to stay euhydrated throughout successive training and competition bouts. Future studies should aim to use polysomnography with the KSD to help validate results found in the questionnaire and take neurological measurements to validate the sleep and fatigue patterns found in this study. Given the dynamic nature of hydration state and many other contributing factors to sleep health and quality over days and prolonged time periods, there is also an opportunity pursue future research designs that include variations in the hydration state (i.e., hypohydrated, euhydrated) over longer periods on sleep quality on multiple subsequent nights and fluctuating multiple cycles of dehydration, rehydration to varying degrees to isolate the effects of hydration state on multiple nights of sleep. It is likely that the effects also have lag or kinetics of effects not yet understood and the extension of an experimental study on greater numbers of subjects will support greater depth of studies in future work.
Data Availability
No datasets were generated or analysed during the current study.
Code Availability
Not applicable.
Abbreviations
Karolinska Sleep Diary
VAS:Visual Analog Scale
USG:Urine Specific Gravity
EUH:Euhydrated state
DEH:Dehydrated state
AD:Ad libitum drinking
SD:Standard Deviation
SE:Standard Error
References
Sawka MN, et al. Exercise and fluid replacement. Med Sci Sports Exerc. 2009;39(2):377–90.
Secher M, Ritz P. Hydration and cognitive performance. J Nutr Health Aging. 2012;16(4):325–9.
Adams WM, et al. Impact of exercise-induced dehydration on perceived sleep quality. FASEB J. 2018;32:4–905.
Armstrong LE, Muñoz CX, Armstrong EM. Distinguishing low and high water consumers—a paradigm of disease risk. Nutrients. 2020;12(3):858.
Melander O. Vasopressin, from regulator to disease predictor for diabetes and cardiometabolic risk. Ann Nutr Metab. 2016;68(Suppl. 2):24–8.
Muñoz CX, et al. Habitual total water intake and dimensions of mood in healthy young women. Appetite. 2015;92:81–6.
Perrier E, et al. Hydration biomarkers in free-living adults with different levels of habitual fluid consumption. Br J Nutr. 2013;109(9):1678–87.
Scott JP, McNaughton LR, Polman RC. Effects of sleep deprivation and exercise on cognitive, motor performance and mood. Physiol Behav. 2006;87(2):396–408.
Liu Y., 2016 Prevalence of healthy sleep duration among adults—United States, 2014. MMWR. Morbidity and mortality weekly report. 65
Wirz-Justice A, Van den Hoofdakker RH. Sleep deprivation in depression: what do we know, where do we go? Biol Psychiat. 1999;46(4):445–53.
Mullington JM, et al. Cardiovascular inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009;51(4):294–302.
Ju Y-ES, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology—a bidirectional relationship. Nat Rev Neurol. 2014;10(2):115–9.
Pross N, et al. Influence of progressive fluid restriction on mood and physiological markers of dehydration in women. Br J Nutr. 2013;109(2):313–21.
Aristotelous P, et al. Effects of controlled dehydration on sleep quality and quantity: a polysomnographic study in healthy young adults. J Sleep Res. 2019;28(3):e12662.
Leiper JB, Molla AM. Effects on health of fluid restriction during fasting in Ramadan. Eur J Clin Nutr. 2003;57(2):S30–8.
Faris ME, et al. Effect of diurnal fasting on sleep during Ramadan: a systematic review and meta-analysis. Sleep and Breathing. 2020;24:771–82.
Roky R, et al. Sleep during Ramadan intermittent fasting. J Sleep Res. 2001;10(4):319–27.
Armstrong LE, et al. Progression of human subjective perceptions during euhydration, mild dehydration, and drinking. Physiol Behav. 2021;229:113211.
Armstrong LE, et al. Inputs to thirst and drinking during water restriction and rehydration. Nutrients. 2020;12(9):2554.
Sekiguchi Y, et al. Countermovement jump, handgrip, and balance performance change during euhydration, mild-dehydration, rehydration, and ad libitum drinking. J Exerc Sci Fit. 2022;20(4):335–9.
Åkerstedt T, et al. The subjective meaning of good sleep, an intraindividual approach using the Karolinska Sleep Diary. Percept Mot Skills. 1994;79(1):287–96.
Armstrong LE, et al. Urinary indices of hydration status. Int J Sport Nutr Exerc Metab. 1994;4(3):265–79.
Ganio MS, et al. Mild dehydration impairs cognitive performance and mood of men. Br J Nutr. 2011;106(10):1535–43.
Armstrong LE, et al. Mild dehydration affects mood in healthy young women. J Nutr. 2012;142(2):382–8.
Maughan R, Shirreffs S, Watson P. Exercise, heat, hydration and the brain. J Am Coll Nutr. 2007;26(sup5):604S-612S.
Keklund G, Åkerstedt T. Objective components of individual differences in subjective sleep quality. J Sleep Res. 1997;6(4):217–20.
Westerlund A, et al. Relationships between questionnaire ratings of sleep quality and polysomnography in healthy adults. Behav Sleep Med. 2016;14(2):185–99.
Rosinger AY, et al. Short sleep duration is associated with inadequate hydration: cross-cultural evidence from US and Chinese adults. Sleep. 2019;42(2):zsy210.
Crispim CA, et al. The influence of sleep and sleep loss upon food intake and metabolism. Nutr Res Rev. 2007;20(2):195–212.
Finch GM, et al. Appetite changes under free-living conditions during Ramadan fasting. Appetite. 1998;31(2):159–70.
Miller HJ. Dehydration in the older adult. J Gerontol Nurs. 2015;41(9):8–13.
Santillanes G, Rose E. Evaluation and management of dehydration in children. Emergency Medicine Clinics. 2018;36(2):259–73.
D’Anci KE, Constant F, Rosenberg IH. Hydration and cognitive function in children. Nutr Rev. 2006;64(10):457–64.
Howe AS, Boden BP. Heat-related illness in athletes. Am J Sports Med. 2007;35(8):1384–95.
Funding
This study was funded by the Human Performance Laboratory at the University of Connecticut. The granting agency had no involvement or restriction on publication.
Ethics declarations
Ethics Approval
This study was conducted according to the guidelines laid down in the Declaration of Helsinki. This study was approved by the Institutional Review Board for Human Studies at the University of Connecticut (Protocol no. H17-291, approved on January 30, 2018). All subjects attended an informational meeting outlining the study’s objectives, risks, benefits, time commitment, and procedures, and provided written informed consent.
Consent to Participate
Written informed consent was obtained from all participants in accordance with the Declaration of Helsinki.
Consent for Publication
The publication of this paper is approved by all authors for the journal of Springer Nature Comprehensive Clinical Medicine.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ky, A.T., Giersch, G.E.W., Sekiguchi, Y. et al. Mild Dehydration by 24-h Fluid Restriction Led to Difficulty Falling Sleeping and Increased Sleep Duration. SN Compr. Clin. Med. 7, 67 (2025). https://doi.org/10.1007/s42399-025-01828-0
Received: 22 August 2024
Revised: 03 March 2025
Accepted: 20 March 2025
Published: 28 March 2025
DOI: https://doi.org/10.1007/s42399-025-01828-0
.png)


![Nobody Is Buying Apple's iPhone Air [video]](https://www.youtube.com/img/desktop/supported_browsers/firefox.png)

![Why Everyone Is Leaving New Zealand [video]](https://news.najib.digital/site/assets/img/broken.gif)
