Popular information: They understood how the immune system is kept in check (pdf)
Populärvetenskaplig bakgrund: De förstod hur immunförsvaret hålls i schack (pdf)

They understood how the immune system is kept in check
The body’s powerful immune system must be regulated, or it may attack our own organs. Mary E. Brunkow, Fred Ramsdell and Shimon Sakaguchi are awarded the Nobel Prize in Physiology or Medicine 2025 for their groundbreaking discoveries concerning peripheral immune tolerance that prevents the immune system from harming the body. Their discoveries have laid the foundation for a new field of research and spurred the development of new treatments, for example for cancer and autoimmune diseases.
The immune system is an evolutionary masterpiece. Every day it protects us from the thousands of different viruses, bacteria and other microbes that attempt to invade our bodies. Without a functioning immune system, we would not survive.
One of the immune system’s marvels is its ability to identify pathogens and differentiate them from the body’s own cells. The microbes that threaten our health do not wear a uniform – they all have different appearances. Many have also developed similarities to human cells, as a form of camouflage. So how does the immune system keep track of what to attack and what to protect? Why doesn’t the immune system attack our bodies more frequently?
 © The Nobel Committee for Physiology or Medicine. Ill. Mattias Karlén
© The Nobel Committee for Physiology or Medicine. Ill. Mattias KarlénResearchers long believed they knew the answer to these questions: that immune cells mature through a process called central immune tolerance. However, our immune system turned out to be more complex than they believed. Mary Brunkow, Fred Ramsdell and Shimon Sakaguchi are awarded the Nobel Prize in Physiology or Medicine 2025 for their discoveries concerning peripheral immune tolerance. The Nobel Prize laureates identified the immune system’s security guards, regulatory T cells, thus laying the foundation for a new field of research. The discoveries have also led to the development of potential medical treatments that are now being evaluated in clinical trials. The hope is to be able to treat or cure autoimmune diseases, provide more effective cancer treatments and prevent serious complications after stem cell transplants.
Let us set the stage for this year’s Nobel Prize in Physiology or Medicine and begin with a short presentation of what researchers knew about the immune system’s T cells in the 1990s. These, our vital protectors, are at the heart of our story.
T cells – essential players in the body’s defence
Helper T cells constantly patrol the body. If they discover an invading microbe, they alert other immune cells, which then mount an attack.
Killer T cells eradicate cells which have been infected by a virus or other pathogens. They can also attack tumour cells.
In addition to these, there are other immune cells with different functions. However, we will not pay these any attention, because in this story the T cells are taking centre stage.
Sensors that can discover invaders
All T cells have special proteins called T-cell receptors on their surface. These receptors can be likened to a type of sensor. Using them, T cells can scan other cells to discover whether the body is under attack. T-cell receptors are special because, like jigsaw pieces, they all have different shapes. They are built from many genes that are randomly combined. In theory, this means the body could make more than 1015 different T-cell receptors.
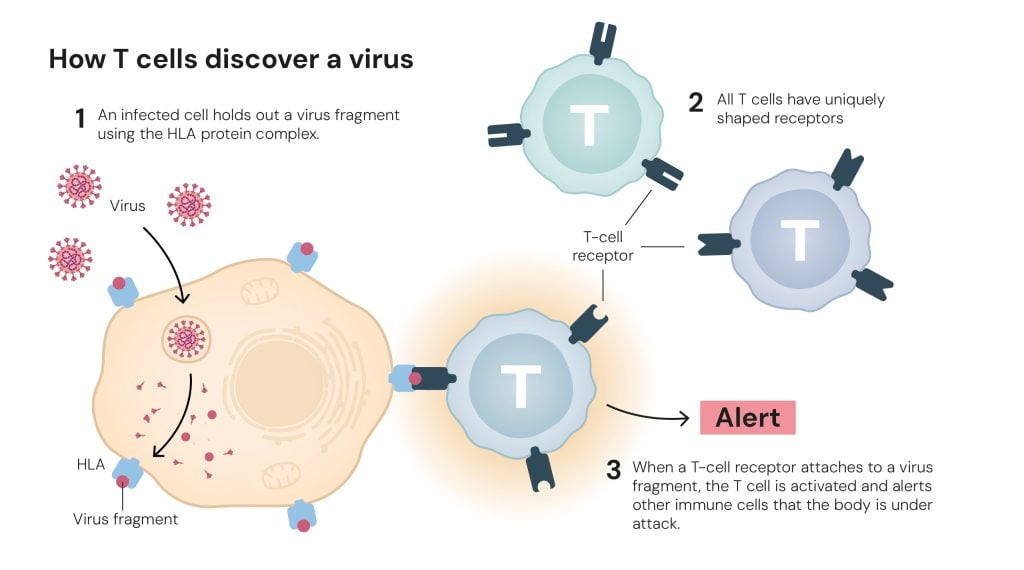 Figure 2. How T cells discover a virus © The Nobel Committee for Physiology or Medicine. Ill. Mattias Karlén
Figure 2. How T cells discover a virus © The Nobel Committee for Physiology or Medicine. Ill. Mattias KarlénThe vast number of T cells with different receptors ensures that there are always some that can detect the shape of an invading microbe (figure 2), including new viruses, such as the one that started the COVID-19 pandemic in 2019. However, the body inevitably also creates T-cell receptors that can attach to parts of the body’s own tissues. So, what makes the T cells react to hostile microbes but not our own cells?
T cells that recognise the body’s own tissue are eliminated
In the 1980s, researchers understood that when T cells mature in the thymus, they undergo a type of test that eliminates the T cells that recognise the body’s own – endogenous – proteins (figure 3). This selection process is called central tolerance.
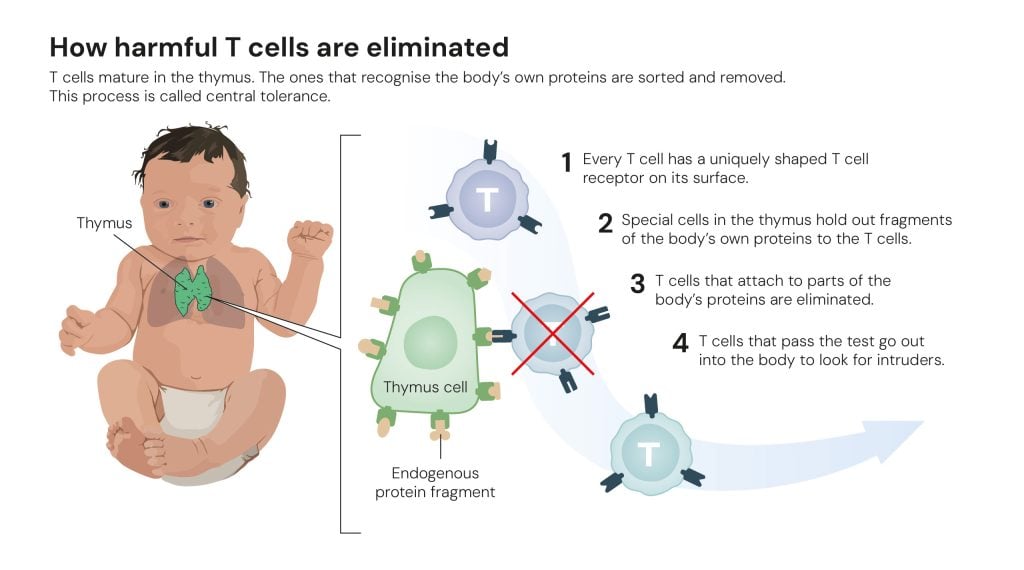 Figure 3. How harmful T cells are eliminated © The Nobel Committee for Physiology or Medicine. Ill. Mattias Karlén
Figure 3. How harmful T cells are eliminated © The Nobel Committee for Physiology or Medicine. Ill. Mattias KarlénIn addition to this, some researchers suspected the existence of a type of cell that they called suppressor T cells. They believed that these dealt with T cells that had slipped through the test in the thymus. However, a few researchers in this field drew far-fetched conclusions from their experiments. When it became apparent that some of the evidence for suppressor T cells was false, researchers rejected the entire hypothesis, and the research field was more or less abandoned.
However, one researcher swam against the tide. His name is Shimon Sakaguchi, and he worked at the Aichi Cancer Center Research Institute in Nagoya, Japan.
Sakaguchi’s insight: the immune system must have a security guard
Shimon Sakaguchi was inspired by an earlier and contradictory experiment performed by his colleagues. To understand the role of the thymus in T cell development, they had surgically removed this organ from newborn mice. They hypothesised that the mice would develop fewer T cells and have a weaker immune system. However, if the operation took place three days after the mice were born, the immune system went into overdrive and ran amok, resulting in the mice developing a range of autoimmune diseases.
To better understand this phenomenon, at the start of the 1980s Shimon Sakaguchi isolated T cells that had matured in genetically identical mice and injected them into the mice without a thymus. This had an interesting effect: there appeared to be T cells that could protect the mice from autoimmune diseases (figure 4).
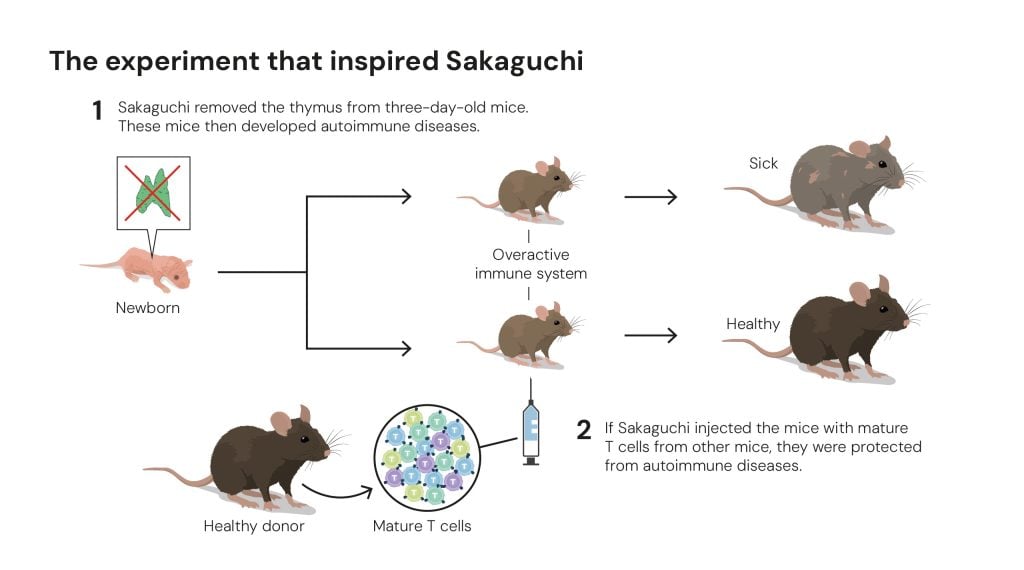 Figure 4. The experiment that inspired Sakaguchi © The Nobel Committee for Physiology or Medicine. Ill. Mattias Karlén
Figure 4. The experiment that inspired Sakaguchi © The Nobel Committee for Physiology or Medicine. Ill. Mattias KarlénThis and other similar results convinced Sakaguchi that the immune system must have some form of security guard, one that calms down other T cells and keeps them in check. But what type of cell was this?
Sakaguchi discovers a new class of T cells
When researchers differentiate between T cells, they use proteins located on the cells’ surface. Helper T cells can be recognised thanks to a protein called CD4, while killer T cells are distinguished by CD8.
In the experiment in which Sakaguchi protected the mice from autoimmune diseases, he used cells with CD4 on their surface – helper T cells. Ordinarily, these cells wake up the immune system and set it to work, but in Sakaguchi’s experiment the immune system was held back. His conclusion was that there must be different forms of T cells that carry CD4.
To test his hypothesis, Sakaguchi needed to find a way of differentiating between the various types of T cell. This took him over a decade, but in 1995 he presented an entirely new class of T cells to the world. In The Journal of Immunology he demonstrated that these T cells – which calm the immune system – are characterised not only by carrying CD4 on their surface, but also a protein called CD25 (figure 5).
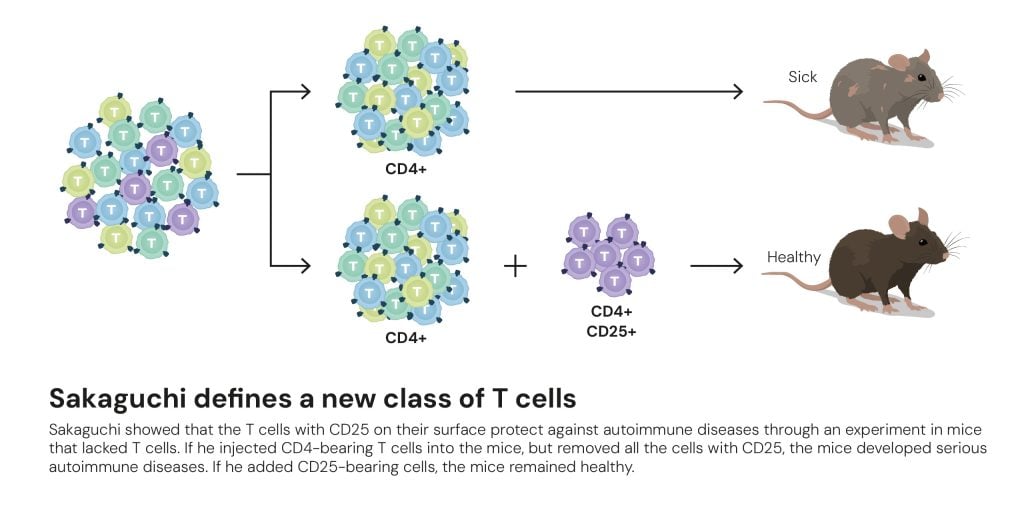 Figure 5. Sakaguchi defines a new class of T cells © The Nobel Committee for Physiology or Medicine. Ill. Mattias Karlén
Figure 5. Sakaguchi defines a new class of T cells © The Nobel Committee for Physiology or Medicine. Ill. Mattias KarlénThis newly identified T cell class was named regulatory T cells. However, many researchers were sceptical about its existence; they wanted more proof before they would believe in Sakaguchi’s discovery. Key information was to come from Mary Brunkow and Fred Ramsdell. It is time for the second act of 2025’s Nobel Prize in Physiology or Medicine. It opens with the birth of sickly male mice in a US laboratory in the 1940s.
A mutation causes mutiny in the immune system
In this laboratory, located in Oak Ridge, Tennessee, researchers were studying the consequences of radiation. Their work was part of the Manhattan Project and the development of the atomic bomb. The mouse strain that plays a vital role in this year’s Nobel Prize was an evolutionary fluke – some male mice were unexpectedly born with scaley and flaky skin, an extremely enlarged spleen and lymph glands, and they lived for just a few weeks.
The mouse strain – which was given the name scurfy – captured the researchers’ attention. Molecular genetics was in its infancy, but they realised that the mutation that caused this disease must be located on the mice’s X chromosome. Half of all the male mice are diseased, but the females can live with this mutation because they have two X chromosomes, of which one has healthy DNA. The female mice thus pass on the scurfy mutation to new generations.
In the 1990s – when molecular tools had become considerably sharper – researchers began to investigate why the male scurfy mice got so ill. It turned out that their organs were being attacked by T cells that destroyed the tissues. For some reason, the scurfy mutation appeared to cause a rebellion in the immune system.
Brunkow and Ramsdell search for explanations for autoimmune diseases
Two of the researchers who became interested in the scurfy mutation were Mary Brunkow and Fred Ramsdell. They worked at a biotech company, Celltech Chiroscience, in Bothell, Washington, US. The company developed pharmaceuticals for autoimmune diseases, and Brunkow and Ramsdell realised that the scurfy mice could provide them with important clues in their work. If they were able to understand the molecular mechanism underlying the mice’s disease, they could gain decisive insights into how autoimmune diseases arise. So, they made a crucial decision: they would search for the scurfy mice’s mutant gene.
Today it is possible to map a mouse’s entire genome and find a mutated gene in a few days. In the 1990s, it was like looking for a needle in a gigantic haystack. The string of DNA that forms the X chromosome in mice consists of around 170 million base pairing nucleotides. Finding a mutation in this mass of DNA was possible, but required time, patience and a creative use of that era’s tools for molecular biology.
Brunkow and Ramsdell find the needle in the DNA haystack
Mapping had shown that the scurfy mutation must be somewhere in the middle of the X chromosome. Brunkow and Ramsdell succeeded in narrowing down the potential area to around 500,000 nucleotides. Then they took on the enormous work of mapping that area of the X chromosome in detail.
This took a long time. When Brunkow and Ramsdell finished, they had established that the area contained 20 potential genes. Their next challenge was to compare these genes in healthy mice and scurfy mice. They examined gene after gene. It was only with the twentieth and final gene that they could shout bingo. After years of dedicated work, they had finally found the scurfy mutation (figure 6).
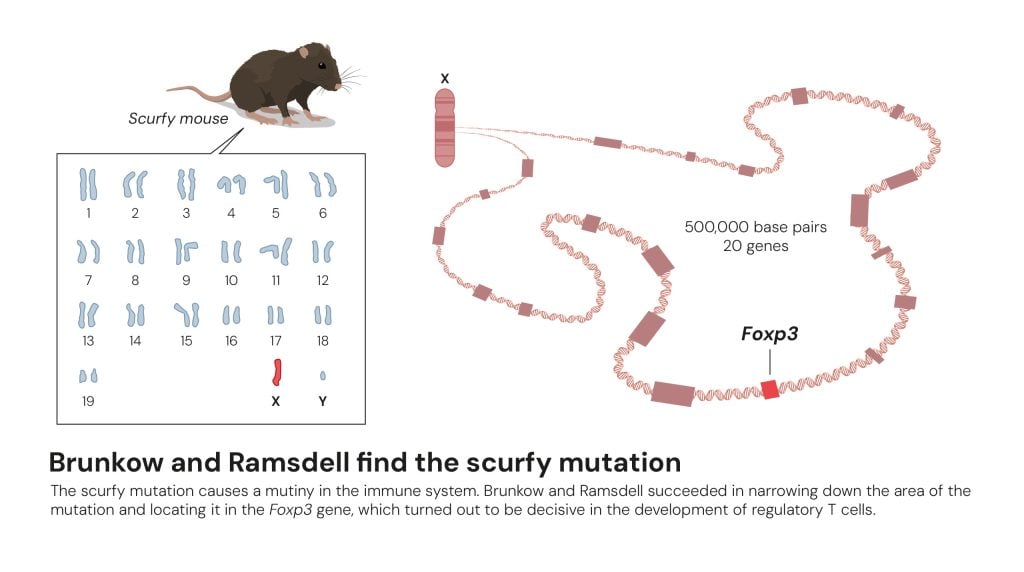 Figure 6. Brunkow and Ramsdell find the scurfy mutation © The Nobel Committee for Physiology or Medicine. Ill. Mattias Karlén
Figure 6. Brunkow and Ramsdell find the scurfy mutation © The Nobel Committee for Physiology or Medicine. Ill. Mattias KarlénThe faulty gene was previously unknown, but had many similarities with a group of genes called forkhead box or FOX genes. These regulate the activity of other genes, which can affect cell development. Mary Brunkow and Fred Ramsdell named the new gene Foxp3.
Their discovery revealed the cause of a serious disease in humans
During their work, Brunkow and Ramsdell had begun to suspect that a rare autoimmune disease, IPEX, which is also linked to the X chromosome, might be the human variant of the scurfy mice’s disease. On searching a database where researchers store information on newly discovered genes, they found the human equivalent of Foxp3. Helped by paediatricians from around the world, they collected samples from boys affected by IPEX. When they mapped the samples, they did indeed find harmful mutations in the FOXP3 gene.
In 2001, in Nature Genetics, Mary Brunkow and Fred Ramsdell revealed that mutations in the FOXP3 gene cause both the human disease called IPEX and the scurfy mice’s ill health. These key findings led to febrile activity in several laboratories. When researchers pieced the puzzle together, they understood that the FOXP3 gene could be important for the regulatory T cells discovered by Sakaguchi.
Regulatory T cells – the body’s security guards
Two years later, Shimon Sakaguchi – and soon other researchers – could convincingly prove that the FOXP3 gene controls the development of regulatory T cells. These cells prevent other T cells from mistakenly attacking the body’s own tissue (figure 7), which is important for a process called peripheral immune tolerance. Regulatory T cells also ensure the immune system calms down after it has eliminated an invader, so it does not continue working at top speed.
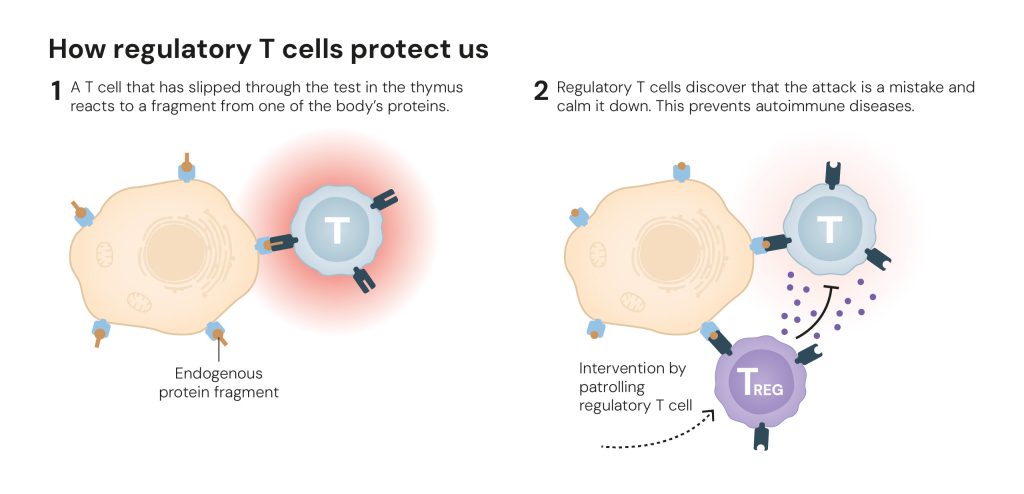 Figure 7. How regulatory T cells protect us © The Nobel Committee for Physiology or Medicine. Ill. Mattias Karlén
Figure 7. How regulatory T cells protect us © The Nobel Committee for Physiology or Medicine. Ill. Mattias KarlénThe fundamental knowledge that researchers have gained through the discovery of regulatory T cells and their importance for peripheral immune tolerance, has spurred the development of potential new medical treatments. Mapping of tumours shows that they can attract large numbers of regulatory T cells that protect them from the immune system. Researchers are therefore trying to find ways to dismantle this wall of regulatory T cells, so the immune system can access the tumours.
In autoimmune diseases, researchers are instead trying to promote the formation of more regulatory T cells. In pilot studies, they are giving patients interleukin-2, a substance that makes regulatory T cells thrive. Researchers are also investigating whether interleukin-2 can be used to prevent organs being rejected after transplantation.
Another strategy researchers are testing to slow an overactive immune system is to isolate regulatory T cells from a patient and multiply them in a laboratory. These are then returned to the patient, who will thus have more regulatory T cells in their body. In some cases, researchers also modify the T cells, putting antibodies on their surface that function like an address label. This allows researchers to send these cellular security guards to a transplanted liver or kidney, for example, and protect the organ from being attacked by the immune system.
There are many more examples of how researchers are testing how regulatory T cells can be used to combat diseases. Through their revolutionary discoveries, Mary Brunkow, Fred Ramsdell and Shimon Sakaguchi have provided fundamental knowledge of how the immune system is regulated and kept in check. They have thus conferred the greatest benefit to humankind.
Mary E. Brunkow, born 1961. Ph.D. from Princeton University, Princeton, USA. Senior Program Manager at the Institute for Systems Biology, Seattle, USA.
Fred Ramsdell, born 1960. Ph.D. 1987 from the University of California, Los Angeles, USA. Scientific Advisor, Sonoma Biotherapeutics, San Francisco, USA.
Shimon Sakaguchi, born 1951. M.D. 1976 and Ph.D. 1983 from Kyoto University, Japan. Distinguished Professor at the Immunology Frontier Research Center, Osaka University, Japan.
Key publications
Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor a-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995:155:1151-1164.
Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001:27:68-73.
Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, Bricarelli FD, Byrne G, McEuen M, Proll S, Appleby M, Brunkow M. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001:27:18-20.
Benne; CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001:27:20-21.
Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003:299:1057-1061.
Science Editors: Gunilla Karlsson Hedestam, Olle Kämpe, Thomas Perlmann, Per Svenningsson, Marie Wahren-Herlenius and Anna Wedell, the Nobel Committee for Physiology or Medicine
Text: Ann Fernholm
Translation: Clare Barnes
Illustrations: Mattias Karlén
©The Nobel Assembly at Karolinska Institutet
This year’s Nobel Prize announcements will take place 6–13 October. All announcements will be streamed live here on nobelprize.org.
Select the category or categories you would like to filter by
Select the category or categories you would like to filter by
Physics
Chemistry
Medicine
Literature
Peace
Economic Sciences
Choose a year you would like to search in
.png)



