Introduction
Liquid brines have yet to be directly observed on Mars, despite some indirect evidence suggesting their potential presence1,2. The extreme cold and dryness of the Martian surface inhibits brine formation through processes like freezing, evaporation, or boiling3. However, brines with extremely low eutectic points—such as those containing calcium perchlorate or mixtures of salts—may remain thermodynamically stable for brief periods, especially at high latitudes and low elevations.
Brine formation on Mars requires both stability against its cold, arid environment and a water source. This can happen either by melting when salts encounter water ice4, or through deliquescence5,6, where salts absorb water vapor. While deliquescence is theoretically possible due to the presence of atmospheric water vapor, its effectiveness is hindered by low vapor pressure and slow reaction rates in Mars’ cold conditions7, which may not be compatible with the diurnal cycle. Ice, abundant in high latitudes and detected by several missions, lies mostly below the surface in regions with atmospheric equilibrium8. This subsurface ice lacks seasonal replenishment, so any potential brines would eventually evaporate in a single episode.
Winter frost presents a more accessible, seasonal ice source, first observed by Viking Lander 2 in Utopia Planitia as H2O frost layers9,10, ranging from tens of microns to millimeter in thickness9,11,12,13. Viking detected frost appearance around Ls ~240° and its abrupt disappearance between solar longitude Ls ~ 330° and Ls ~360°. H2O and CO2 frost have since been detected at polar latitudes by the Phoenix lander14,15, or by remote sensing16,17,18,19 while dedicated campaigns within the Mars Science Laboratory20 and Mars 202021 missions did not unambiguously identify frost. In this paper I hypothesized that the physical contact between frost and salts at the surface allows for the brief formation of transient brines.
Although the Viking landers couldn’t directly detect salts, other instruments have inferred their presence, including sulfates and perchlorates, which were later confirmed by Phoenix and other rovers22,23. And retrospectively some of the original life experiments carried by Viking 2 have been reinterpreted as the result of reactions with perchlorate24. These findings indicate that small amounts of salts, including perchlorates and chlorates, are likely widespread in the regolith at Viking’s landing site, making it a unique location to study frost-salt interactions.
Brine stability on Mars depends on three main processes: freezing, which is influenced by temperature; boiling, which is influenced by the total atmospheric pressure; and evaporation, driven by water vapor partial pressure. In this paper I use the meteorological data returned by the Viking lander 225 to constrain the nature and stability of brines formed in contact with frost. In the cold Martian winter, any liquid formation would occur within the stability range of ice. Mars’ average surface pressure of about 6 mbar places the boiling point of water at 0 °C, making brines more stable in low-altitude areas like impact basins, where higher pressures can raise the boiling point slightly3. Brines also have a lower saturation vapor pressure due to reduced water activity, which further supports their stability.
Frost and brines stability
At the Viking 2 landing site, pressures varied between ~7.5 and 10 mbar, allowing a boiling point just above 0 °C. During frost observations, air temperatures ranged from 155 K to 220 K, far below the triple point of pure liquid water, preventing frost from melting into pure liquid. The frost sublimated well before reaching the summer peak temperatures, which were still around 20 K below water’s triple point. Even the maximum ground temperatures modeled using the Global Circulation Model (GCM) from the Laboratoire de Météorologie Dynamique (LMD) and compiled into the Mars Climate Database (MCD), only increased the maximum temperature by 10 K, compared to the air temperatures from Viking 2 measurements, far below the melting temperature of pure ice. However, in these low temperature conditions, any brine that could form would be stable against boiling.
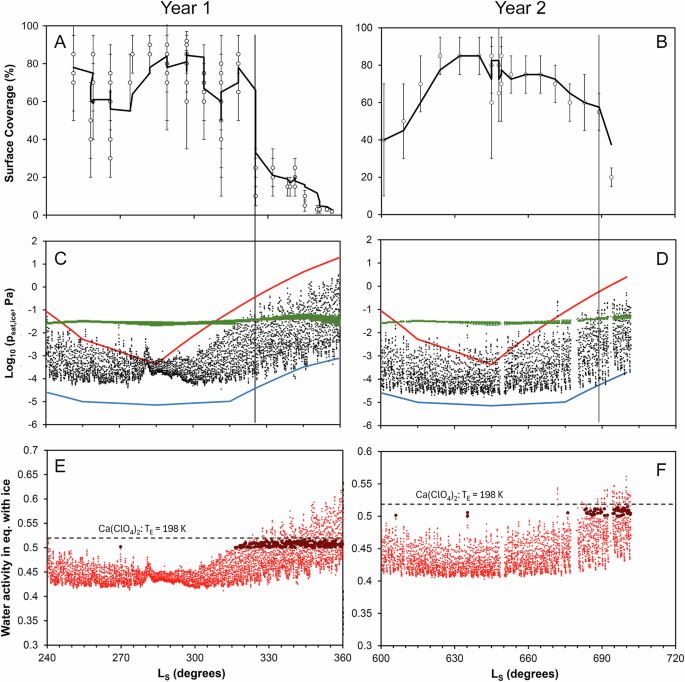
To determine frost sublimation timing, surface coverage from Viking lander images12 was compared with the saturation vapor pressure of ice, calculated from measured and modeled temperatures (Fig. 1). Sublimation occurs when the saturation vapor pressure of ice exceeds the atmospheric water vapor pressure, calculated using the water mixing ratio modeled using the LMD-GCM. The frost coverage in year 1 dropped sharply from around 70% to less than 40% at Ls ~ 320°, and was completely gone by Ls ~ 360° (Fig. 1A). The sudden drop at Ls ~ 320° coincides with sublimation of frost, defined when the saturation vapor pressure above the frost, determined from Viking 2’s surface temperatures, exceeds the GCM-modeled atmospheric water pressure (Fig. 1C). If considering the maximum GCM-modeled ground temperatures, then sublimation occurs earlier around Ls ~ 305-310°, because modeled ground temperatures are a few K above measured temperatures. In year 2, fewer measurements were available, but the frost reduction at Ls ~ 690° is also in the range of the column abundances (Fig. 1B, D).
Figure A, B show frost surface coverage determined from Viking images12, for years 1 and 2, respectively. The thin vertical lines indicate the very rapid sublimation of water frost, as shown by the surface coverage in the lander images. Figure C, D show water vapor saturation pressure with respect to ice for years 1 and 2, calculated using temperature measurements by Viking 2 (black dots). The blue and red curves show the minimum and maximum surface temperatures, respectively, modeled using the LMD-GCM. The green dots show the water vapor partial pressure in the atmosphere, also determined using the GCM. As soon as the ice vapor pressure is larger than the atmospheric water partial pressure, ice starts to sublimate. The Viking data suggest sublimation started around Ls 315°, but if the surface reached the maximum temperature suggested by the GCM model, sublimation could have started as early as Ls ~305-310°. Figure E, F show the water activity of the brine in equilibrium with frost, calculated using measured air temperatures and Eqs. (1), (2). The dark red larger dots represent all the data for which the water activity with respect to ice (calculated from the measured temperatures) is above 0.5 and simultaneously the atmospheric relative humidity is also above 0.5, thus ensuring that the brine is thermodynamically stable. A strong argument for the formation of perchlorate brines is that regardless of the lower boundary on water activity, the maximum value where both water activity and atmospheric humidity are above 0.5 is 0.52, which is exactly the eutectic of calcium perchlorate (identified by the horizontal dashed lines).
Stability against freezing
Concentrated brines exhibit lower freezing temperatures because the activity of water is lowered by electrochemical interactions between water and ions in solution, which depends on the nature and concentration of ions. Thus, several brines have eutectic temperatures equal to or lower than 220 K3, but only for salts composed of either calcium or magnesium and chloride, chlorate or perchlorate anions as counterparts. Calcium chloride (CaCl2, TE = 220 K) represents the upper boundary, while calcium perchlorate (Ca(ClO4)2) marks the lower temperature boundary (TE = 198 K). However, for most of the winter the temperatures are too cold even for Ca-perchlorate to form a brine. Air temperatures reach maximum values of about 200 K (210 K for ground temperatures) around Ls = 330°, which is the time at which the frost starts sublimating and the frost surface coverage significantly decreases (Fig. 1A, B). Nonetheless, it takes from Ls = 330° to 360° for the frost to completely disappear. Therefore, Ca-perchlorate brines could potentially form through frost melting in this transition zone. Previous work has determined that the formation of perchlorate brines through deliquescence is favored during the recession of the seasonal ice cap and subsequent sublimation of exposed water26, resulting in warmer temperatures and higher local vapor pressures.
Beside temperature, brine formation also depends on the liquid’s saturation vapor pressure (psat_liq). This is crucial for two reasons: first, psat_liq varies with water activity in the liquid, and secondly, a water-based brine remains stable below the water’s triple point if its vapor pressure is no higher than that of ice at the same temperature. Thus, the ratio of psat_ice (Eq. (1)) to psat_liq (Eq. (2)) indicates the required water activity for the brine to be in equilibrium with ice. Converting measured temperatures to equilibrium water activity, I found that winter values remain mostly below 0.52 (Fig. 1E, F), meaning a brine composed of Ca-perchlorate would remain frozen until temperatures rise to its eutectic point (198 K), around Ls = 330° in year 1 and Ls = 690° in year 2.
Stability against evaporation
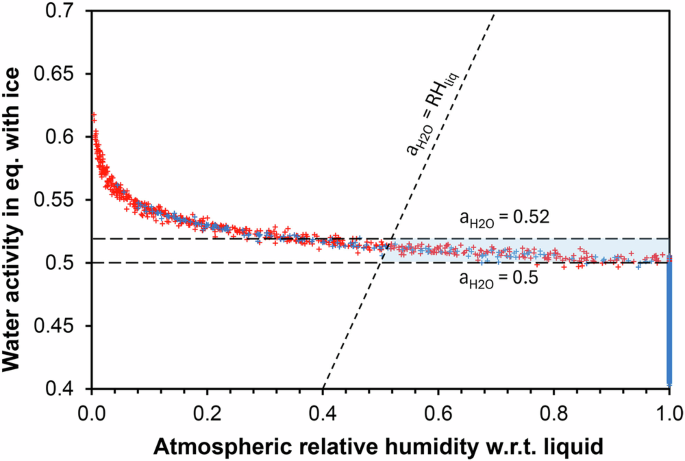
The third process limiting the stability of brines is evaporation, which occurs when the saturation vapor pressure above the brine is higher than the water vapor pressure in the atmosphere. The gradient of water pressure between the surface and the atmosphere drives the evaporation process. A lower activity of water in the brine results in a lower water vapor pressure, which prevents or limits evaporation. Therefore, the second condition for the brine to be stable is if its water activity is below the atmospheric relative humidity. I compared the water activity of the brine, calculated from measured air temperatures, to the atmospheric relative humidity (Fig. 2). All the data align on a curve showing a weak anticorrelation between the water activity and the atmospheric humidity. The range of values is much larger for atmospheric relative humidity (from almost 0 to 1) while the water activity in equilibrium with ice ranges from 0.5-0.6. According to the conditions for liquid to be stable, the water activity of the brine must be equal or lower than the water activity in equilibrium with ice, and the water activity must also be inferior to the relative humidity in the atmosphere (Fig. 2). I considered two boundaries for the water activity: 0.52 for pure Ca-perchlorate and a lower boundary of 0.5 if perchlorates are mixed with other salts (chlorides or chlorates), based on multiple studies on binary, ternary and multi-phase equilibria27. The results show that a significant portion of the data exhibit stable brine conditions (Fig. 2).
Brines are only stable if their water activity is below the equilibrium value with ice and below the atmospheric water humidity. Therefore, these circumstances are illustrated by the blue region. The diagonal line represents the equality between water activity and atmospheric humidity, thus the minimum atmospheric humidity values for brine to remain stable. The two horizontal dashed lines represent two values for brine activity: 0.52 which is the eutectic value of calcium perchlorate (and appears very slightly above the stability curve) and a water activity of 0.5 which accounts for possible mixtures with other salts such as chlorate or chloride, and which appears completely stable in the liquid phase.
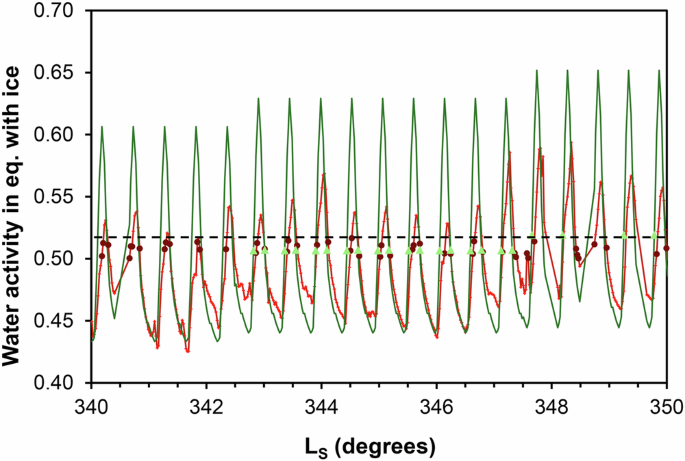
Within the exception of a few isolated points, most conditions under which brines are stable occur in a very narrow band of water activity between 0.5 and 0.52 and for Ls values above 315° in year 1 and 680° in year 2 (Fig. 1E, F). No water activity value goes above the eutectic of Ca-perchlorate, therefore showing that Ca-perchlorate is most likely responsible for the formation of brines (when the brine forms, the water activity or temperature remains at the eutectic of Ca-perchlorate). Finally, I compare the water activity between the measured air temperatures (1.6 m above ground) and the modeled ground temperatures over a short timescale during the sublimation of frost, i.e. from Ls = 340° to 350° (Fig. 3). Due to the higher maximum temperature, the surface can reach high water activity values (0.6–0.65) compared to the air measurements (0.52–0.58). However, the stability zone for brines (0.5–0.52) remains unaffected and if the brines formed in slightly higher temperatures, they would still be limited by the 0.52 Ca-perchlorate eutectic. These very limited conditions only occur late in the winter, when frost starts sublimating, i.e., above Ls = 315° in year 1 and above Ls = 680° in year 2.
The dark red dots and light green triangles represent all the data for which the water activity in equilibrium with ice is above 0.5 and simultaneously the atmospheric relative humidity is also above 0.5, for measured and modeled temperatures, respectively. Despite the significantly higher maximum diurnal temperatures, the modeled water activities also remain under 0.52 (horizontal dashed line).
Conclusions
This study, using combined Viking 2 meteorological data and modeling, shows that brines may briefly form on Mars as frost sublimates, with Ca-perchlorate likely driving this process. Brines appear during frost sublimation as temperatures rise, and vanish when frost is fully gone or when temperatures exceed 198 K. Although the resulting brine volumes are likely minimal (a fraction of the limiting compound between ice and perchlorate), this seasonal mechanism suggests frost-rich areas as promising sites for future astrobiological exploration and potential habitability on Mars. Of course, these results are heavily dependent on the modeled data. The difference of temperature between those measured by Viking 2 and the MCD surface model (a few K for the maximum values), despite having a significant effect on saturation pressure, did not significantly change the overall results regarding brine formation, nature and stability. Alternatively, the atmospheric water abundance close to the surface could fluctuate more significantly than the MCD modeled data I used, due to localized adsorption, and diffusion within the regolith (and thus porosity or tortuosity). Further models could explore more realistic scenarios of surface water vapor in equilibrium with the regolith and / or brines.
These findings provide a new perspective on the transient presence of liquid water on Mars, a key factor in assessing planetary habitability. The confirmation that brines can form at the Viking 2 landing site during late winter—albeit for short periods—suggests that similar processes may occur in other frost-bearing regions, especially at mid-to-high latitudes19. This challenges the prevailing assumption that modern Mars is entirely devoid of liquid water, reinforcing the importance of perchlorate chemistry in extending the stability range of water under Martian conditions28. Additionally, the strong correlation between brine formation and seasonal frost cycles highlights specific periods when transient water activity is most likely, which could guide the planning of future astrobiological investigations. Robotic landers equipped with in situ hygrometers and chemical sensors could target these seasonal windows to directly detect brine formation and constrain the timescales over which these liquids persist.
Beyond the immediate implications for habitability, these results refine our understanding of Mars’ current water cycle. By demonstrating that even minimal frost deposits can contribute to transient brine formation, this study suggests that localized microenvironments might support intermittent liquid phases, influencing surface chemistry, regolith weathering, and even slope activity. The methodology applied here—using Viking 2 meteorological data to reconstruct brine stability—could be extended to datasets from more recent missions, such as InSight and Curiosity, to investigate whether similar processes occur in different climatic settings. Moreover, this work underscores the need for future landers to deploy high-resolution imaging and thermal mapping to track frost evolution in real-time, enabling more precise modeling of brine stability and its potential impact on surface morphology.
Methods
In this study, I revisited Viking 2’s meteorological data over two Martian winters, covering periods from Ls ~ 240° to ~360° in year one, and Ls ~ 600° to ~700° in year two, to evaluate whether environmental conditions might have supported transient brine formation. I used the meteorological data measured by Viking 2, which included air temperature, and pressure25,29. Temperatures were measured about 1.6 meters above ground and therefore should be slightly different from actual ground temperatures. Moreover, Viking 2 was not equipped with hygrometers to measure the relative humidity at the surface. Therefore, I used the surface temperatures and atmospheric water vapor mixing ratios determined by the Laboratoire de Météorologie Dynamique (LMD) Global Circulation Model30 and compiled in the Mars Climate Database (MCD, available at https://www-mars.lmd.jussieu.fr/mcd_python/) The water mixing ratios were then converted into water vapor partial pressure. The MCD provides complete diurnal cycles, every hour, and every 5 degrees of solar longitude. It is likely that the water vapor partial pressure follows a diurnal cycle, with variability around one order of magnitude. Phoenix measurements showed that the water vapor pressure varied between ~0.03 and ~0.5 Pa over a diurnal cycle31,32. The modeled values are in the lower range of the Phoenix data (~10−2 Pa), thus providing a lower boundary to brine stability.
Using the temperature data, I calculated both saturation vapor pressure above ice and liquid water, using the two equations below33:
Water pressure above the ice (in bar):
$${p}_{{sat},{ice}}={10}^{-5}\exp \left[9.550426-\frac{5723.265}{T}+3.53068\times {{{\mathrm{ln}}}}\,T-0.0072833\times T\right]$$
(1)
And water pressure above pure liquid water (in bar):
$${p}_{{sat},{liq}}= {10}^{-5}\exp \left[54.842763-\frac{6763.22}{T}-4.210{{{\mathrm{ln}}}}\,T+0.000367\times T\right.\\ \left.+\tanh \left[0.0415\times \left(T-218.8\right)\right]\right.\\ \left. \times \left[53.878-\frac{1331.22}{T}-9.44523{{{\mathrm{ln}}}}T+0.014025\times T\right]\right]$$
(2)
Then the ratio psat_ice/psat_liq gives the water activity in equilibrium with ice. The ratio of pH2O/psat_liq gives the atmospheric relative humidity (pH2O being the atmospheric water vapor pressure).
Data availability
The raw Viking 2 meteorological data used in this study are available on the NASA Planetary Data System Atmosphere Data Node at https://pds-atmospheres.nmsu.edu/cgi-bin/getdir.pl?dir=data&volume=vl_1001. The data packages used were vl_tbin.DAT, vl_tbin.LBL and vl_tbin.XML. The Laboratoire de Météorologie Dynamique (LMD) Mars Climate Databse is available at this link: https://www-mars.lmd.jussieu.fr/mars/access.html. The interpreted data used in the figures and results is available on the Zenodo website34.
References
Renno, N. O. et al. Possible physical and thermodynamical evidence for liquid water at the Phoenix landing site. J. Geophys. Res. 2009, 114. https://doi.org/10.1029/2009JE003362.
Martin-Torres, F. J. et al. Transient liquid water and water activity at Gale crater on Mars. Nat. Geosci. 8, 357–361 (2015).
Chevrier, V. F., Rivera-Valentín, E. G., Soto, A. & Altheide, T. S. Global Temporal and Geographic Stability of Brines on Present-day Mars. Planet. Sci. J. 1, 64 (2020).
Chevrier, V. F., Rivera-Valentin, E. G. Formation of recurring slope lineae by liquid brines on present-day Mars. Geophys. Res. Lett. 2012, 39. https://doi.org/10.1029/2012GL054119.
Gough, R. V., Chevrier, V. F., Baustian, K. J., Wise, M. E. & Tolbert, M. A. Laboratory studies of perchlorate phase transitions: Support for metastable aqueous perchlorate solutions on Mars. Earth Planet Sci. Lett. 312, 371–377 (2011).
Gough, R. V., Chevrier, V. F., Tolbert, M. A. Deliquescence of Perchlorate/chloride Mixtures: Implications for Stable and Metastable Aqueous Solutions on Mars. Lunar and Planetary Science Conference 2012, XLIII.
Fischer, E., Martínez, G. M., Elliott, H. M. & Rennó, N. O. Experimental evidence for the formation of liquid saline water on Mars. Geophys. Res. Lett. 41, 2014GL060302 (2014).
Boynton, W. V. et al. Distribution of hydrogen in the near surface of Mars: Evidence for subsurface ice deposits. Science 297, 81–85 (2002).
Hart, H. M. & Jakosky, B. M. Composition and stability of the condensate observed at the Viking Lander 2 site on Mars. Icarus 66, 134–142 (1986).
Wall, S. D. Analysis of condensates formed at the Viking 2 lander site - The first winter. Icarus 47, 173–183 (1981).
Higuchi, K. Formation of frost layer of water ice on Mars. Icarus 154, 181–182 (2001).
Svitek, T. & Murray, B. Winter frost at Viking Lander 2 site. J. Geophys. Res. 95, 1495–1510 (1990).
Clark, R. N. The surface condensates on Mars observed by Viking: Frost layers several tenth of a millimeter thick. Lunar Planet. Sci. Conf. XI, 155–156 (1980).
Smith, P. H. et al. H2O at the Phoenix landing site. Science 325, 58–61 (2009).
Whiteway, J. A. et al. Mars water-ice clouds and precipitation. Science 325, 68–70 (2009).
Carrozzo, F., Bellucci, G., Altieri, F., D’aversa, E. & Bibring, J.-P. Mapping of water frost and ice at low latitudes on Mars. Icarus 203, 406–420 (2009).
Schorghofer, N. & Edgett, K. S. Seasonal surface frost at low latitudes on Mars. Icarus 180, 321–334 (2006).
Kelly, N. J. et al. Seasonal polar carbon dioxide frost on Mars: CO2 mass and columnar thickness distribution. J. Geophys. Res.: Planets 111, https://doi.org/10.1029/2006JE002678 (2006, 2007).
Valantinas, A. et al. Evidence for transient morning water frost deposits on the Tharsis volcanoes of Mars. Nat. Geosci. 17, 608–616 (2024).
Martínez, G. et al. Likely frost events at Gale crater: Analysis from MSL/REMS measurements. Icarus 280, 93–102 (2016).
Martínez, G. M. et al. The First Frost Detection Campaign by the Mars 2020 Perseverance Rover: Implementation and Results. 54th Lunar and Planetary Science Conference 2023; 2023; 2023. p. 2184.
Hecht, M. H. et al. Detection of Perchlorate and the Soluble Chemistry of Martian Soil at the Phoenix Lander Site. Science 325, 64–67 (2009).
Kounaves, S. P. et al. Identification of the perchlorate parent salts at the Phoenix Mars landing site and possible implications. Icarus 232, 226–231 (2014).
Navarro-González, R., Vargas, E., de la Rosa, J., Raga, A. C. & McKay, C. P. Reanalysis of the Viking results suggests perchlorate and organics at midlatitudes on Mars. J. Geophys. Res. 115, E12010 (2010).
Hess, S. T., Henry, R. M., Leovy, C. B., Ryan, J. A. & Tillman, J. E. Meteorological results from the surface of Mars: Viking 1 and 2. J. Geophys. Res. 82, 4559–4574 (1977).
Pál, B. D. & Kereszturi, Á Deliquescence probability maps of Mars and key limiting factors using GCM model calculations. Icarus 376, 114856 (2022).
Chevrier, V. F., Fitting, A. B. & Rivera-Valentín, E. G. Limited Stability of Multicomponent Brines on the Surface of Mars. Planet. Sci. J. 3, 125 (2022).
Rivera-Valentín, E. G., Chevrier, V. F., Soto, A. & Martínez, G. Distribution and habitability of (meta) stable brines on present-day Mars. Nat. Astron. 4, 756–761 (2020).
Chamberlain, T., Cole, H., Dutton, R., Greene, G. & Tillman, J. Atmospheric measurements on Mars: The Viking meteorology experiment. Bull. Am. Meteorol. Soc. 57, 1094–1105 (1976).
Forget, F. et al. Improved general circulation models of the Martian atmosphere from the surface to above 80 km. J. Geophys. Res. 104, 24155–124175 (1999).
Rivera-Valentin, E. G. & Chevrier, V. F. Revisiting the Phoenix TECP data: Implications for regolith control of near-surface humidity on Mars. Icarus 253, 156–158 (2015).
Fischer, E., Martínez, G., Rennó, N., Tamppari, L. & Zent, A. Relative humidity on Mars: new results from the Phoenix TECP sensor. J. Geophys. Res. Planets 124, 2780–2792 (2019).
Murphy, D. M. & Koop, T. Review of the vapour pressures of ice and supercooled water for atmospheric applications. Quart. J. R. Meteor Soc. 131, 1539–1565 (2005).
Chevrier, V. F. Viking lander 2 winter meteorological data (Ls 240 to 360) and derived water - brines thermodynamic properties. 1 ed. https://doi.org/10.5281/zenodo.15054166: Zenodo; 2025.
Acknowledgements
I would like to thank Germán Martínez, Bernadett Pál and an anonymous reviewer for their comments which significantly improved the quality of this manuscript. I am also acknowledging funding from the NASA Habitable Worlds grant #80NSSC20K0227.
Ethics declarations
Competing interests
The author declares no competing interests.
Peer review
Peer review information
Communications Earth & Environment thanks Bernadett Pál, Germán Martínez and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Candice Bedford and Joe Aslin. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chevrier, V.F. Perchlorate brine formation from frost at the Viking 2 landing site. Commun Earth Environ 6, 447 (2025). https://doi.org/10.1038/s43247-025-02411-0
Received: 20 November 2024
Accepted: 23 May 2025
Published: 10 June 2025
DOI: https://doi.org/10.1038/s43247-025-02411-0
.png)