Main
It is estimated that nearly half of AD cases worldwide are attributable to modifiable risk factors1,2,3. Physical inactivity, in particular, plays a prominent role in the USA and Europe1,2. Although animal studies support a benefit of physical activity on AD progression4,5, important knowledge gaps remain in human literature, limiting the effective translation into AD prevention trials. Most existing observational studies rely on self-reported physical activity6,7,8,9,10,11,12, which is prone to recall bias and misreporting, especially in populations with or at risk for cognitive impairment. In addition, most studies focused on the clinical syndrome of AD7,9,12,13,14, with few directly examining core AD biomarkers15,16,17,18,19,20,21, and even fewer have done so longitudinally22,23. A clearer understanding of how objectively measured physical activity is associated with the progression of AD neuropathology is essential, particularly during the preclinical period, when there is likely the greatest potential to modify disease trajectory.
With the increasing popularity of digital wearables, daily step count has become an easily accessible and understood measure of physical activity. Emerging literature suggests that higher step counts are linked to lower all-cause mortality24,25. Our previous study demonstrated that higher step counts in cognitively unimpaired (CU) older adults with elevated baseline beta-amyloid (Aβ) burden were associated with slower prospective cognitive decline26, supporting a potential protective role of physical activity in the preclinical stage of AD. However, it remains unknown whether this protective association with cognition is mediated by differences in AD neuropathology burden, or what levels of physical activity are associated with slower cognitive decline.
The current study addressed these questions by leveraging an expanded Harvard Aging Brain Study (HABS) cohort of CU older individuals with pedometer-measured physical activity, longitudinal Aβ and tau positron emission tomography (PET) data, and annual cognitive assessments up to 14 years. We examined whether physical activity is associated with slower cognitive and functional decline through different rates of Aβ and tau accumulation. We further examined the dose–response associations with physical activity levels to help inform future AD prevention trials and public health policies.
Results
Participant characteristics and longitudinal trajectories
The current study examined 296 participants from HABS who were CU at baseline and followed longitudinally27. Cognition and function were assessed annually using the Preclinical Alzheimer’s Cognitive Composite-5 (PACC5) and Clinical Dementia Rating28 Sum of Boxes (CDR-SOB) scores, respectively. Global Aβ and inferior temporal cortex (ITC) tau burdens were measured longitudinally in a subset of participants (Aβ n = 241; tau n = 172), with participants undergoing their first tau scan at 2.2 ± 1.5 years after baseline because the technique was introduced mid-study. Table 1 summarizes the participant characteristics and longitudinal follow-up. The unadjusted longitudinal trajectories for global Aβ, ITC tau, PACC5 and CDR-SOB are shown in Extended Data Fig. 1. For illustration purposes only, to more clearly visualize the individual trajectories, the data were plotted according to high versus low baseline Aβ burden in columns and physical activity (pedometer-measured mean steps per day) in rows, defined by above and below the median values.
Baseline associations with physical activity
We first examined the associations between physical activity and baseline demographics, imaging and cognitive variables. There was a negative association between physical activity and age (Spearman’s r = −0.27, P < 0.001). Males had greater physical activity than females (Sex [males compared to females]: β = 927 [95% confidence intervals 247 to 1,607], P = 0.008). There were no significant associations between physical activity and education (rpartial = 0.08, P = 0.16), or baseline Aβ burden (rpartial = −0.004, P = 0.95), adjusting for age and sex. There was a significant interaction between lower baseline physical activity and elevated Aβ on greater initial ITC tau burden, adjusting for age, sex, years of education and time interval between study baseline and first tau scan (Physical activity × Aβ: β = −0.19 [−0.30 to −0.08], P = 0.001) (Extended Data Fig. 2). There was no additional independent association between baseline physical activity and initial ITC tau burden (Physical activity: β = −0.06 [−0.18 to 0.05], P = 0.28). Importantly, there were no interactive or independent associations of initial Aβ and tau burden with baseline physical activity, adjusting for the same covariates (Aβ × ITC tau: β = −0.04 [−0.15 to 0.07], P = 0.44; Aβ: β = 0.10 [−0.07 to 0.28], P = 0.25; ITC tau: β = 0.01 [−0.21 to 0.22], P = 0.96) (Extended Data Fig. 3). There were also no significant interactive (Physical activity × Aβ) or independent effects of physical activity on baseline PACC5 (Physical activity × Aβ: β = 0.003 [−0.07 to 0.08], P = 0.93; Physical activity: β = 0.003 [−0.07 to 0.08], P = 0.92). Baseline associations with CDR-SOB were not examined because participants were clinically unimpaired at baseline.
Physical activity associations with longitudinal Aβ
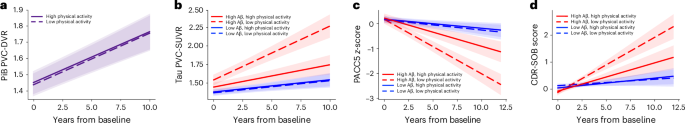
For primary analyses, we examined the effects of baseline physical activity on longitudinal imaging and cognitive measures. Using a linear mixed effects model, we found no association between baseline physical activity and longitudinal Aβ burden (Physical activity × Time: β = −0.0006 [−0.01 to 0.01], P = 0.92) (Fig. 1a).
a, Linear mixed effects model revealed no association between baseline physical activity and longitudinal Aβ burden (β = −0.0006 [−0.01 to 0.01], P = 0.92; n = 241). b–d, By contrast, there were significant interactions between baseline physical activity and Aβ burden on longitudinal ITC tau burden (b), longitudinal cognition measured with PACC5 (c) and longitudinal functional decline measured with CDR-SOB scores (d). Individuals with high baseline physical activity and elevated Aβ (solid red line) showed slower ITC tau accumulation (β = −0.13 [−0.19 to −0.06], P < 0.001; n = 172) (b), slower PACC5 decline (β = 0.10 [0.05 to 0.16], P < 0.001; n = 296) (c) and slower CDR-SOB progression (β = −0.14 [−0.22 to −0.05], P = 0.001; n = 296) (d). Statistical significance was assessed using two-tailed t-tests, with P < 0.05 considered statistically significant without adjustment for multiple comparisons. Baseline physical activity (mean steps per day) and Aβ burden were modeled as continuous variables. To visualize the model results, the estimated trajectories based on representative levels of low versus high baseline physical activity and (for tau, PACC5 and CDR-SOB models) low versus high baseline Aβ burden are presented, with error bands representing 95% confidence intervals for the estimated trajectories. Low and high physical activity are represented by −1 and +1 s.d. relative to the mean (low, 2,800 steps per day; high, 8,700 steps per day). Low and high Aβ are represented, for illustration purposes, by the mean Aβ burden of Aβ-negative (PiB PVC-DVR = 1.17) and Aβ-positive (PiB PVC-DVR = 1.85) participants, respectively. The numbers of participants contributing longitudinal data to each 2.5-year segment for the respective statistical models are summarized in Extended Data Table 5.
Physical activity–Aβ interactions on longitudinal tau, cognition
We then examined the interactive associations between baseline physical activity and Aβ burden on longitudinal cognition and function. Baseline Aβ was modeled as a continuous variable to leverage the full dynamic range of global Aβ burden29. Consistent with our earlier work26, this larger HABS sample with extended follow-up showed a significant physical activity by Aβ interaction on longitudinal PACC5, whereby higher physical activity was associated with slower Aβ-related PACC5 decline (Physical activity × Aβ × Time: β = 0.10 [0.05 to 0.16], P < 0.001; Fig. 1c). In addition, there was an independent association between higher physical activity and slower PACC5 decline (Physical activity × Time: β = 0.06 [0.004 to 0.12], P = 0.04). We further extended our previous findings and identified a significant physical activity by Aβ interaction on longitudinal CDR-SOB, whereby higher physical activity was associated with slower Aβ-related functional decline (Physical activity × Aβ × Time: β = −0.14 [−0.22 to -0.05], P = 0.001) (Fig. 1d). There was no additional main effect of physical activity on CDR-SOB progression (Physical activity × Time: β = −0.05 [−0.13 to 0.04], P = 0.29).
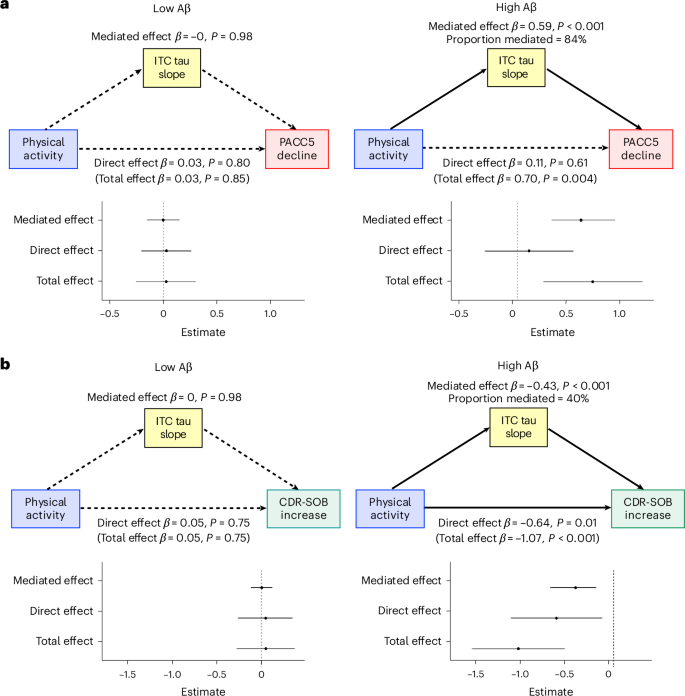
We then addressed the question of whether the associations between physical activity and cognitive and functional decline were mediated by differences in tau pathology. We found a concordant interaction, whereby higher baseline physical activity was associated with attenuated Aβ-related ITC tau accumulation (Physical activity × Aβ × Time: β = −0.13 [−0.19 to −0.06], P < 0.001) (Fig. 1b). There was additionally an independent association between higher physical activity and slower tau accumulation (Physical activity × Time: β = −0.07 [−0.13 to −0.003], P = 0.04). Moderated mediation analysis demonstrated that slower ITC tau accumulation fully mediated the association between higher physical activity and slower PACC5 decline in individuals with elevated baseline Aβ (mediated effect: β = 0.59 [0.32 to 0.91], P < 0.001; direct effect: β = 0.11 [−0.30 to 0.52], P = 0.61; 84% mediated) (Fig. 2a). For CDR-SOB, slower ITC tau accumulation partially mediated the association between higher physical activity and slower CDR-SOB progression in individuals with elevated baseline Aβ (β = −0.43 [−0.71 to −0.20], P < 0.001; 40% mediated) (Fig. 2b). There remained a significant direct effect of physical activity on functional decline (β = −0.64 [−1.16 to −0.13], P = 0.01). In individuals with low baseline Aβ, there were no significant total or mediated effects of physical activity on PACC5 decline or CDR-SOB progression (P > 0.75).
a,b, Individual slopes for ITC tau, PACC5 (a) and CDR-SOB (b) were extracted from linear mixed effects models for moderated mediation analyses (n = 172; in participants who have both longitudinal tau and cognitive data). We modeled physical activity (mean steps per day) as predictor, ITC tau slope as mediator and PACC5 or CDR-SOB slopes as outcome. Both physical activity and Aβ burden were modeled as continuous variables in the mediation models. For the moderation analyses, low and high levels of Aβ burden were represented by the mean Aβ burden of Aβ-negative (PiB PVC-DVR = 1.17) and Aβ-positive (PiB PVC-DVR = 1.85) participants, respectively. Statistical testing was performed using a quasi-Bayesian Monte Carlo method based on 10,000 simulations to generate the estimates and 95% confidence intervals, with two-tailed P < 0.05 considered statistically significant without adjustment for multiple comparisons. Results demonstrated that at an elevated level of baseline Aβ burden, slower ITC tau accumulation fully mediated the association between higher physical activity and slower PACC5 decline (β = 0.59 [0.32 to 0.91], P < 0.001, 84% mediated) (a) and partially mediated the association between higher physical activity and slower CDR-SOB progression (β = −0.43 [−0.71 to −0.20], P < 0.001, 40% mediated) (b). In individuals with low baseline Aβ burden, there were no significant total or mediated effects of physical activity on PACC5 decline (total effect: β = 0.03 [−0.27 to 0.31], P = 0.85; mediated effect: β = −0.002 [−0.16 to 0.15], P = 0.98) or CDR-SOB progression (total effect: β = 0.05 [−0.28 to 0.37], P = 0.75; mediated effect: β = 0.001 [−0.11 to 0.12], P = 0.98). The error bars represent the 95% confidence intervals for the estimated mediated, direct and total effects.
Sensitivity analyses
The interaction between baseline physical activity and Aβ on longitudinal tau, PACC5 and CDR-SOB remained significant in sensitivity analyses assessing for possible confounds (Table 2): (1) using a more stringent physical activity cutoff excluding days that registered fewer than 1,000 steps; (2) using Aβ and tau PET data without partial volume correction (PVC); (3) including only participants age <70 years to minimize potential confounding effects of advanced age (the negative association between physical activity and age was absent in this age <70 subgroup; Spearman’s r = −0.01, P = 0.93); (4) adjusting for the season of physical activity measurement, self-reported physical activity, systemic vascular risk and depressive symptoms; (5) excluding participants who developed mild cognitive impairment or dropped out of the study within the first 2 years of follow-up; and (6) adjusting for baseline tau, PACC or CDR-SOB in their respective models to account for physical activity effects on baseline outcome measures.
Physical activity levels on Aβ-related changes in tau, cognition
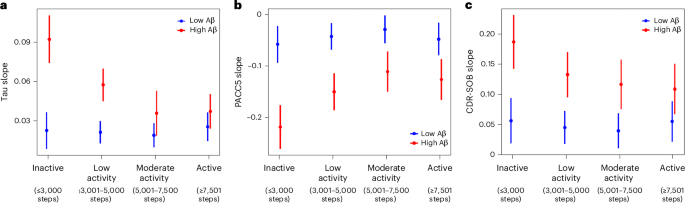
Lastly, we examined the dose–response associations between levels of physical activity and longitudinal tau accumulation, cognitive and functional decline. Ordinal levels of physical activity were defined using cutoffs adapted from ref. 30, which were modified to better suit the lower activity levels in older adults and to ensure an adequate number of participants in each subgroup (Extended Data Table 1): ‘inactive’ (≤3,000 per day), ‘low activity’ (3,001–5,000 steps per day), ‘moderate activity’ (5,001–7,500 steps per day) and ‘active’ (≥7,501 steps per day). Participant characteristics across physical activity levels are summarized in Extended Data Table 2. Higher physical activity levels were associated with younger age, but baseline demographics, imaging and cognitive variables were not significantly different across physical activity groups adjusting for age and sex. In linear mixed effects models using the inactive subgroup as reference, all higher levels of physical activity were associated with slower Aβ-related ITC tau accumulation, PACC5 decline and CDR-SOB progression, except that the slower CDR-SOB progression in the low activity group did not reach statistical significance (Table 3). To better visualize the dose–response relationships, we modeled the physical activity level by Aβ interaction on extracted ITC tau, PACC5 and CDR-SOB slopes (Fig. 3). In individuals with elevated baseline Aβ, even low levels of physical activity (3,001–5,000 steps per day) were associated with substantially slower rates of tau accumulation, cognitive and functional decline. There were further attenuations of tau accumulation and cognitive and functional decline at moderate activity (5,001–7,500 steps per day), with similar rates in the active group (≥7,501 steps per day), suggesting a plateauing of effects.
a–c, Using extracted slopes for ITC tau (n = 172) (a), PACC5 (b) and CDR-SOB (c) (PACC5 and CDR-SOB, n = 296), we examined the interactive effects of baseline physical activity level (ordinal) and Aβ burden (continuous) using linear regression models. Levels of physical activity (ordinal) were defined as inactive (≤3,000 steps), low activity (3,001–5,000 steps), moderate activity (5,001–7,500 steps) and active (≥7,501 steps). The number of individuals in each physical activity subgroup included in the tau, PACC5 and CDR-SOB analyses are summarized in Extended Data Table 1. Aβ burden was modeled as a continuous variable. For illustration purposes, low and high Aβ are represented by the mean Aβ burden of Aβ-negative (PiB PVC-DVR = 1.17) and Aβ-positive (PiB PVC-DVR = 1.85) participants, respectively. The error bars represent the 95% confidence intervals for the estimated effects of physical activity levels on tau and cognitive slopes at representative levels of low and high Aβ burden. Results demonstrate that in individuals with elevated baseline Aβ, even low levels of physical activity (3,001–5,000 steps) were associated with substantially slower rates of tau accumulation and cognitive decline compared to inactive individuals. There were further attenuations of tau accumulation and cognitive and functional decline at moderate activity (5,001–7,500 steps per day), with similar rates in the active group (≥7,501 steps per day).
To help contextualize the associations between physical activity levels and cognitive and functional decline, we used the linear mixed effects models from Table 3 to estimate the longitudinal PACC5 and CDR-SOB trajectories at representative levels of low versus high baseline Aβ burden (represented by the mean global Aβ burden of Aβ-negative and Aβ-positive participants, respectively) across physical activity levels (Extended Data Fig. 4). For individuals with elevated Aβ, from baseline to the median cognitive follow-up of 9 years, estimated PACC5 z-scores declined by 2.5 (inactive), 1.5 (low activity), 1.1 (moderate activity) and 1.2 (active) points, respectively. Compared to inactive individuals, cognitive decline was lower by 40%, 54% and 51% across increasing physical activity levels. Over the same period, estimated CDR-SOB scores increased by 2.2 (inactive), 1.4 (low activity), 1.2 (moderate activity) and 1.1 (active) points in individuals with elevated Aβ. Compared to inactivity, there were 34%, 45% and 51% slower functional decline across increasing physical activity levels. Using −1.5 PACC5 z-score as a threshold for impaired cognition31, CU individuals with elevated Aβ were estimated to reach this threshold for cognitive worsening at 6.5 (inactive), 9.6 (low activity), 13.6 (moderate activity) and 12.7 (active) years from baseline, respectively. Similarly, using a CDR-SOB increase of 1.5 as a threshold for clinically meaningful functional decline in early AD32, individuals with elevated Aβ were estimated to reach this threshold at 7.1 (inactive), 10.2 (low activity), 11.9 (moderate activity) and 13.6 (active) years from baseline, respectively.
Discussion
In an expanded cohort of 296 CU older adults with pedometer-measured physical activity, baseline Aβ PET and up to 14 years of annual cognitive follow-up, we replicated our previous finding of a protective association between higher physical activity and slower Aβ-related cognitive decline, and further demonstrated a similar relationship with functional decline. Importantly, we demonstrated that these associations with cognition and function were not related to differences in cross-sectional or longitudinal Aβ pathology. Instead, in a subset of 172 individuals with longitudinal tau PET, we found a new and concordant association between higher physical activity and slower Aβ-related early neocortical tau accumulation, which significantly mediated the relationships with slower cognitive and functional decline. Our dose–response analyses further suggest that the largest incremental differences between increasing physical activity levels were observed in the most sedentary individuals, with plateauing associations with both tau and cognition at a moderate level of activity (5,001–7,500 steps per day), potentially offering a more approachable physical activity goal for older sedentary individuals. Collectively, our results support targeting physical inactivity, either alone or in combination with anti-Aβ therapy, as an intervention in future prevention trials to slow the progression of tau pathology and delay the onset of cognitive and functional decline in preclinical AD.
Many observational studies have demonstrated associations between physical activity and reduced risks of cognitive impairment and AD. However, most existing literature relied on self-reported physical activity, which is susceptible to recall bias or misreporting, especially among populations with or at risk for cognitive impairment. A few longitudinal studies have examined the effects of objectively measured physical activity (by pedometer or accelerometer) in older adults without dementia and found associations with reduced risks of cognitive decline, AD or all-cause dementia13,33,34,35. Our previous study26 extended these findings to CU individuals and demonstrated an association between higher step counts and slower prospective cognitive decline in preclinical AD. The current study replicated this finding using a considerably larger cohort, including younger individuals in late midlife, and over a substantially longer duration of follow-up (median of 9.3 years instead of 6 years). The latter is notable given that some observational studies found diminished benefits of physical activity with longer follow-up time36,37,38. We further expanded our cognitive findings and demonstrated a similar association with slower Aβ-related functional decline measured by CDR-SOB, which has been frequently used as an outcome measure in AD clinical trials39,40.
There is growing interest in understanding whether physical activity associations with reduced cognitive decline are mediated by differences in AD neuropathology, with most studies focusing on Aβ. Although animal models suggest that physical exercise may reduce Aβ burden4,41, evidence in human studies has been mixed. A few cross-sectional studies found associations between higher self-reported physical activity and lower Aβ pathology15,16,17,18,19,20. Other large CU cohort studies have found no associations between self-reported physical activity and cross-sectional or longitudinal Aβ PET burden21,22,23. Consistent with the latter, our study found no associations between pedometer-measured physical activity and baseline or longitudinal Aβ burden, suggesting that our findings were unlikely to be driven by different rates of Aβ accumulation.
By contrast, we demonstrated a new relationship between physical activity and longitudinal tau burden, where higher step counts were associated with slower early neocortical tau accumulation in individuals with elevated baseline Aβ. We further demonstrated that reduced tau accumulation significantly mediated the associations between higher physical activity and slower cognitive decline in preclinical AD. These observational findings support a potential role for physical activity in modifying the AD pathological cascade, which remains to be confirmed in future randomized clinical trials. Interestingly, although tau change fully mediated the effects of PACC5 decline (84% mediated), it only partially mediated (40%) the effects on CDR-SOB progression, with a significant remaining direct effect. This suggests that a substantial portion of the physical activity association with slower functional decline was mediated by mechanisms besides tau-related injury, which should be further examined in future studies (for example, physical frailty and functional reserve).
A small number of prior studies have investigated the cross-sectional associations between physical activity and tau. A few have found associations between higher physical activity and lower cerebrospinal fluid phosphorylated tau or phosphorylated tau to Aβ42 ratios19,20,42,43. Others have examined the cross-sectional relationships between self-reported physical activity and tau PET with mixed results. Two studies found that higher self-reported physical activity was associated with lower cross-sectional tau burden44,45. However, a larger study of CU individuals found no cross-sectional associations between self-reported physical activity and regional tau burden and no significant mediation of physical activity effects on cross-sectional cognition by tau21. It is likely that our use of objectively measured physical activity and, more importantly, our longitudinal design increased our sensitivity to detect a relationship between physical activity and early aggregated tau pathology in preclinical AD.
Although our rich longitudinal PET and cognitive data are a substantial strength, this study remains observational, and we are unable to rule out potential reverse causality (that is, individuals with prodromal AD may have reduced physical activity from their underlying disease). However, several factors reduce this likelihood. First, there were no associations between physical activity (independently or interactively with Aβ) and baseline cognition. Although there was a physical activity and Aβ interaction on baseline tau burden, a reversed relationship was not observed—higher cross-sectional Aβ or tau burden (individually or interactively) was not associated with lower baseline physical activity. In addition, the association between higher physical activity and slower longitudinal ITC tau accumulation remained significant after adjusting for the effects of lower initial tau burden. Our findings were further unchanged in sensitivity analyses that excluded participants who developed mild cognitive impairment (or dropped out) within the first 2 years of follow-up, reducing the likelihood that our findings were driven by individuals with impending cognitive and/or clinical changes. Although these observations are reassuring, it is important to emphasize that randomized clinical trials are ultimately needed to establish causal relationships between physical activity and preclinical AD progression.
There is substantial public interest in understanding how much physical activity is needed to improve health. Although this question cannot be directly answered by observational studies, we examined the dose–response associations between physical activity levels and progression of tau and cognitive decline, which may help inform the design of future AD prevention trials. Our results suggest that even a modest increase in physical activity may be associated with attenuated tau accumulation and cognitive decline in sedentary individuals on a preclinical AD trajectory: compared to inactive individuals, low levels of physical activity were associated with 34% to 40% slower cognitive and functional decline over 9 years (the median duration of cognitive follow-up). Notably, the associations with more favorable tau and cognitive trajectories reached a plateau by moderate levels of physical activity (5,001–7,500 steps per day), which may be a less daunting goal for sedentary older adults than the popular goal of 10,000 steps per day commonly referenced in lay media. Several studies have demonstrated a similar curvilinear relationship between step count and all-cause mortality or dementia24,25,34, with benefits plateauing around 6,000–8,000 steps per day for older adults and around 8,000–10,000 steps per day for younger populations, suggesting a ceiling effect. Specifically for the current study, our findings suggest that there may be a limit to how much increasing physical activity may dampen the detrimental effects of Aβ on tau and cognitive and functional decline, and support designing clinical trials that combine lifestyle interventions with anti-Aβ therapy to more effectively modify the trajectory of preclinical AD.
Future studies are needed to understand the mechanisms underlying the protective effects of physical activity on Aβ-related tau pathology and cognitive decline. Although adjusting for composite vascular risk did not impact our findings, better vascular fitness remains a likely potential mechanism. Greater physical activity has been linked to better cardiopulmonary fitness46,47, which has, in turn, been shown to dampen the negative association between Aβ and cognition48. In addition, greater accelerometer physical activity in CU older adults has been associated with greater cerebral blood flow49, which has been shown to be impaired early in the AD cascade50. Lastly, greater physical activity and/or exercise have also been shown in animal models and humans to decrease inflammation and upregulate VEGF-A, BDNF and Irisin51,52,53,54,55, which are candidate pathways with the potential to modulate Aβ effects on tau and cognition56,57,58,59,60.
The current study has several limitations. As discussed above in greater detail, this is an observational study and we are unable to fully rule out the potential influence of reverse causality. Future randomized clinical trials are required to establish causal relationships. Although our pedometer-measured physical activity is a strength, it was only assessed at baseline and did not classify the duration, intensity or history of physical activity, nor capture nonstep-related physical activity (for example, swimming, resistance training). Future studies with longitudinal physical activity measurements (for example, with actigraphy) and life course physical activity data are needed to better delineate temporal trends. The HABS cohort consists of highly educated, predominantly non-Hispanic white individuals and excluded those with symptomatic cerebrovascular disease and poorly controlled vascular risk factors at baseline, which may limit the generalizability of our findings to other populations.
In summary, leveraging a deeply phenotyped CU cohort with objectively measured physical activity and longitudinal neuroimaging and cognitive assessments, we demonstrated an association between higher physical activity and attenuated accumulation of early neocortical tau pathology, which significantly mediated the relationship with slower cognitive and functional decline in preclinical AD. Our dose–response analyses further suggest that the incremental differences in associations between higher physical activity levels and slower tau and cognitive changes were greatest for the most sedentary individuals, and appeared to reach a plateau by moderate levels of physical activity (5,001–7,500 steps per day). Taken together, our findings support targeting physical inactivity as a strategy in future randomized clinical trials to modify the trajectory of tau and cognition in preclinical AD, and potentially provide an easily understood and more attainable physical activity goal for older sedentary individuals at high risk of cognitive decline. Our results further suggest that AD prevention trials using activity-based interventions may benefit from preferentially enrolling sedentary individuals with elevated Aβ levels, longer trial durations, and consideration of examining biomarker endpoint with tau PET, because these approaches may maximize the likelihood of demonstrating a protective effect of physical activity on early AD progression.
Methods
Participants
The current study included 296 participants from HABS27, a community cohort of individuals aged 50 to 90 who were CU at baseline (global CDR28 of 0, education-adjusted Mini-Mental State Examination61 score of 27 or greater, and Logical Memory IIa Delayed Recall performance in the normal range62). Inclusion and exclusion criteria and study protocol have been detailed previously27 and published online (https://www.synapse.org/Synapse:syn53910452/wiki/631007). We examined all eligible participants with baseline physical activity measurement, Aβ PET imaging and at least two longitudinal cognitive assessments. Extended Data Fig. 5 illustrates that of 348 eligible HABS participants at baseline, 50 were excluded from the current study because of missing data. Importantly, the included group did not differ in demographics or baseline cognition (Extended Data Table 3). Baseline characteristics by self-reported sex are summarized in Extended Data Table 4. Additional subsets of participants were used in the longitudinal Aβ PET (n = 241) and tau PET (n = 172) analyses (Table 1). Data were collected from April 2010 to February 2025. The Mass General Brigham Institutional Review Board approved the HABS protocol and procedures, and all participants signed a written informed consent before the completion of any study procedures.
Physical activity
Baseline physical activity was measured using a waistband-mounted pedometer (HJ-720ITC; Omron Healthcare), which has been shown to accurately measure step counts63. Participants were asked to wear the pedometer for seven consecutive days during waking hours. Using previously published cutoffs for pedometer data quality, days that registered <100 or >30,000 steps were excluded26,64. We included participants with at least 4 days of recorded activity within these cutoffs64. Mean steps per day were used as the primary measure of physical activity, which was square-root transformed to account for skewness.
PET imaging
Detailed methods have been published previously65. Briefly, global Aβ burden was measured using PiB PET as a DVR across a composite of frontal, lateral temporal and parietal, and retrosplenial regional uptake defined using FreeSurfer (v.6.0). 18F-Flortaucipir PET was introduced mid-study to measure tau, with participants undergoing their first 18F-flortaucipir PET at 2.2 ± 1.5 years after baseline. Tau was measured as standardized uptake value ratio in the ITC, a prominent site of early neocortical tau accumulation in preclinical AD associated with emerging cognitive impairment65. Both Aβ and tau PET used cerebellar gray matter as reference and the geometric transfer matrix method for PVC66. Sensitivity analyses were performed using PET data without PVC.
Cognitive assessment
Cognition was assessed using the PACC5 (ref. 67), a composite z-score that includes the Mini-Mental State Examination61, Wechsler Adult Intelligence Scale-Revised Digit Symbol Coding68, Wechsler Memory Scale-Revised Logical Memory delayed recall62, Free and Cued Selective Reminding Test (free recall plus total recall)69 and Category Fluency Test70. Functional decline was assessed using the CDR-SOB score.
Statistical analyses
We used R v.4.3.1. Continuous variables were z-transformed before analysis to obtain standardized effect sizes, with the exception of PACC5 (already a z-score). For illustrative purposes, nonstandardized variables were used when plotting model results to enhance interpretability (‘ggplot2’ and ‘plot_model’ packages). A two-tailed P < 0.05 was considered statistically significant.
We first examined the associations between baseline physical activity with age and sex. We also examined the associations between physical activity and baseline imaging and cognitive variables, adjusting for age and sex.
For primary analyses, we used linear mixed effects model (‘nlme’ package) to examine: (1) the effects of physical activity on longitudinal Aβ; and (2) the interactive effects of physical activity and baseline Aβ on longitudinal ITC tau, PACC5 and CDR-SOB, respectively. Baseline Aβ was modeled as a continuous variable to leverage the full dynamic range of Aβ burden, which has been shown to capture additional information on preclinical AD progression over a dichotomous approach and avoids variability introduced by positivity threshold selection, which remains an evolving area of research29. Time was operationalized as years from baseline assessment. We adjusted for age, sex, APOE ε4 carrier status, education, their interactions with time, and included random intercepts and slopes. There were no significant APOE ε4 effects on longitudinal tau, PACC5 or CDR-SOB; therefore, APOE was removed from these models to include three participants with missing genotype data.
We conducted the following sensitivity analyses to examine the robustness of our primary findings: (1) using a more stringent physical activity cutoff excluding days that registered <1,000 steps; (2) using PET data without PVC; (3) including only participants age <70 years to minimize potential confounding effects of advanced age; (4) adjusting for additional potential confounders and their interactions with time—season of physical activity measurement, total self-reported physical activity (CHAMPS questionnaire71), systemic vascular risk (office-based Framingham Heart Study cardiovascular disease risk score72) and depressive symptoms (geriatric depression scale); (5) excluding participants who developed mild cognitive impairment (n = 5) or dropped out (n = 11) within the first 2 years of follow-up to reduce the potential for reverse causality; and (6) adjusting for baseline tau, PACC or CDR-SOB in their respective models to account for physical activity effects on baseline outcome measures.
We further used moderated mediation analysis (‘mediation’ package73) to examine whether tau accumulation mediated the interactive effects of physical activity and Aβ on PACC5 decline or CDR-SOB progression. Individual slopes of ITC tau, PACC5 and CDR-SOB change were extracted from unadjusted linear mixed models with time as the only predictor, including both random slopes and intercepts. We conducted moderated mediation analysis with PACC5 or CDR-SOB slope as outcome, ITC tau slope as mediator, baseline Aβ (continuous) as moderator, and adjusted for age, sex, education and time interval between baseline Aβ and first tau scan. To examine for moderation effects by baseline Aβ, mediation models were run at both low and high levels of baseline Aβ, which were represented by the mean Aβ burden of Aβ-negative (PiB PVC-DVR = 1.17) and Aβ-positive (PiB PVC-DVR = 1.85) participants respectively, defined using the conventional Aβ-positivity threshold (PiB PVC-DVR = 1.324). Statistical testing was performed using a quasi-Bayesian Monte Carlo method based on 10,000 simulations to generate the estimates and 95% confidence intervals.
Lastly, we examined the dose–response relationships between the levels of physical activity and associations with Aβ-related tau accumulation and cognitive decline. Ordinal levels of physical activity were defined using cutoffs adapted from ref. 30: inactive (≤3,000 steps per day), low activity (3,001 to 5,000 steps per day), moderate activity (5,001 to 7,500 steps per day) and active (≥7,501 steps per day). Using linear mixed effects models, we examined the interactive effects of baseline physical activity levels (ordinal) and Aβ (continuous) on longitudinal ITC tau, PACC5 and CDR-SOB, adjusting for the same covariates. To contextualize the effects of physical activity on cognitive decline, we used the model results to estimate predicted PACC5 and CDR-SOB trajectories across physical activity levels at representative levels of low versus high baseline Aβ burden, which were represented by the mean global Aβ burden of Aβ-negative (PiB PVC-DVR = 1.17) and Aβ-positive participants (PiB PVC-DVR = 1.85) respectively as described above. We calculated the predicted changes in PACC5 and CDR-SOB scores from baseline to year 9 (median duration of cognitive follow-up) and calculated the percent difference in decline across higher activity levels compared to inactive individuals. Lastly, we used literature-derived thresholds for cognitive impairment (lower than −1.5 PACC5 z-score31) and clinically meaningful functional decline in early AD (increase of 1.5 points on CDR-SOB32) to estimate the time from baseline to these thresholds of cognitive and functional worsening for individuals with elevated Aβ across physical activity levels.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Deidentified data from HABS can be requested online (https://www.synapse.org/habs). Details of the request process have been published previously27 and include the completion of an online data request form and acceptance of the terms of a data use agreement. The approval process is primarily designed to ensure that the proposed purpose of the data request is consistent with the data use agreement and would not pose a risk to the HABS participants. The most important restriction of use is to not attempt to re-identify the participants in any way. Other requirements include agreement to abide by human subject research policies, only using the data for the project detailed in the data request (separate requests can be made for additional projects), no commercialization of the data, and no marketing or fundraising with the data.
Code availability
All statistical analyses and raw figures were generated using R (v.4.3.1), with the following open source packages: car (v.3.1-2), dplyr (v.1.1.2), ggplot2 (v.3.5.1), mediation (v.4.5.0), nlme (v.3.1-162), ppcor (v.1.1), sjPlot (v.2.8.15). No custom code or packages were used in the statistical analyses.
References
Norton, S., Matthews, F. E., Barnes, D. E., Yaffe, K. & Brayne, C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 13, 788–794 (2014).
Nianogo, R. A. et al. Risk factors associated with Alzheimer disease and related dementias by sex and race and ethnicity in the US. JAMA Neurol. 79, 584–591 (2022).
Livingston, G. et al. Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission. Lancet 404, 572–628 (2024).
Moore, K. M. et al. A spectrum of exercise training reduces soluble Aβ in a dose-dependent manner in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 85, 218–224 (2016).
Yang, L. et al. Long-term exercise pre-training attenuates Alzheimer’s disease-related pathology in a transgenic rat model of Alzheimer’s disease. Geroscience 44, 1457–1477 (2022).
Yaffe, K., Barnes, D., Nevitt, M., Lui, L.-Y. & Covinsky, K. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch. Intern. Med. 161, 1703–1708 (2001).
Scarmeas, N. et al. Physical activity, diet, and risk of Alzheimer disease. JAMA 302, 627–637 (2009).
Chang, M. et al. The effect of midlife physical activity on cognitive function among older adults: AGES—Reykjavik Study. J. Gerontol. A Biol. Sci. Med. Sci. 65A, 1369–1374 (2010).
Tolppanen, A.-M. et al. Leisure-time physical activity from mid- to late life, body mass index, and risk of dementia. Alzheimers Dement. 11, 434–443.e6 (2015).
Tan, Z. S. et al. Physical activity, brain volume, and dementia risk: the Framingham Study. J. Gerontol. A Biol. Sci. Med. Sci. 72, 789–795 (2017).
Sabia, S. et al. Physical activity, cognitive decline, and risk of dementia: 28 year follow-up of Whitehall II cohort study. BMJ 357, j2709 (2017).
Ogino, E., Manly, J. J., Schupf, N., Mayeux, R. & Gu, Y. Current and past leisure time physical activity in relation to risk of Alzheimer’s disease in older adults. Alzheimers Dement. 15, 1603–1611 (2019).
Buchman, A. S. et al. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology 78, 1323–1329 (2012).
Najar, J. et al. Cognitive and physical activity and dementia: a 44-year longitudinal population study of women. Neurology 92, e1322–e1330 (2019).
Liang, K. Y. et al. Exercise and Alzheimer’s disease biomarkers in cognitively normal older adults. Ann. Neurol. 68, 311–318 (2010).
Head, D. et al. Exercise engagement as a moderator of the effects of APOE genotype on amyloid deposition. Arch. Neurol. 69, 636–643 (2012).
Brown, B. M. et al. Physical activity and amyloid-β plasma and brain levels: results from the Australian Imaging, Biomarkers and Lifestyle Study of Ageing. Mol. Psychiatry 18, 875–881 (2013).
Müller, S. et al. Relationship between physical activity, cognition, and Alzheimer pathology in autosomal dominant Alzheimer’s disease. Alzheimers Dement. 14, 1427–1437 (2018).
Hou, X.-H. et al. Associations of healthy lifestyles with cerebrospinal fluid biomarkers of Alzheimer’s disease pathology in cognitively intact older adults: the CABLE study. Alzheimers Res. Ther. 13, 81 (2021).
Zhong, S. et al. Associations of physical activity with Alzheimer’s disease pathologies and cognition: the CABLE study. J. Alzheimers Dis. 89, 483–492 (2022).
Aslanyan, V. et al. Protective effects of sleep duration and physical activity on cognitive performance are influenced by β-amyloid and brain volume but not tau burden among cognitively unimpaired older adults. Neuroimage Clin. 39, 103460 (2023).
Pedrero-Chamizo, R. et al. Alzheimer’s disease prevention: apolipoprotein e4 moderates the effect of physical activity on brain beta-amyloid deposition in healthy older adults. J. Sci. Med. Sport 27, 402–407 (2024).
Slee, M. G. et al. Physical activity and brain amyloid beta: a longitudinal analysis of cognitively unimpaired older adults. Alzheimers Dement. 20, 1350–1359 (2024).
Lee, I.-M. et al. Association of step volume and intensity with all-cause mortality in older women. JAMA Intern. Med. 179, 1105–1112 (2019).
Paluch, A. E. et al. Daily steps and all-cause mortality: a meta-analysis of 15 international cohorts. Lancet Public Health 7, e219–e228 (2022).
Rabin, J. S. et al. Associations of physical activity and β-amyloid with longitudinal cognition and neurodegeneration in clinically normal older adults. JAMA Neurol. 76, 1203–1210 (2019).
Dagley, A. et al. Harvard Aging Brain Study: dataset and accessibility. Neuroimage 144, 255–258 (2017).
Morris, J. C. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 43, 2412–2414 (1993).
Farrell, M. E. et al. Association of longitudinal cognitive decline with amyloid burden in middle-aged and older adults: evidence for a dose–response relationship. JAMA Neurol. 74, 830–838 (2017).
Tudor-Locke, C., Johnson, W. D. & Katzmarzyk, P. T. Accelerometer-determined steps per day in US adults. Med. Sci. Sports Exerc. 41, 1384–1391 (2009).
Papp, K. V. et al. Clinical meaningfulness of subtle cognitive decline on longitudinal testing in preclinical AD. Alzheimers Dement. 16, 552–560 (2020).
Lansdall, C. J. et al. Care partner‐informed meaningful change thresholds for the Clinical Dementia Rating‐Sum of Boxes for trials of early Alzheimer’s disease. Alzheimers Dement. 20, 5889–5900 (2024).
Memel, M., Buchman, A. S., Bennett, D. A. & Casaletto, K. Relationship between objectively measured physical activity on neuropathology and cognitive outcomes in older adults: resistance versus resilience? Alzheimers Dement (Amst.) 13, e12245 (2021).
del Pozo Cruz, B., Ahmadi, M., Naismith, S. L. & Stamatakis, E. Association of daily step count and intensity with incident dementia in 78430 adults living in the UK. JAMA Neurol. 79, 1059–1063 (2022).
Nguyen, S. et al. Accelerometer‐measured physical activity and sitting with incident mild cognitive impairment or probable dementia among older women. Alzheimers Dement. 19, 3041–3054 (2023).
Bruijn, R. F. A. G. et al. The association between physical activity and dementia in an elderly population: the Rotterdam Study. Eur. J. Epidemiol. 28, 277–283 (2013).
Floud, S. et al. Body mass index, diet, physical inactivity, and the incidence of dementia in 1 million UK women. Neurology 94, e123–e132 (2020).
Kivimäki, M. et al. Physical inactivity, cardiometabolic disease, and risk of dementia: an individual-participant meta-analysis. BMJ 365, l1495 (2019).
van Dyck, C.H. et al. Lecanemab in early Alzheimer’s disease. N. Engl. J. Med. 388, 9–21 (2023).
Sims, J. R. et al. Donanemab in early symptomatic Alzheimer disease. JAMA 330, 512–527 (2023).
Adlard, P. A., Perreau, V. M., Pop, V. & Cotman, C. W. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J. Neurosci. 25, 4217–4221 (2005).
Law, L. L. et al. Moderate intensity physical activity associates with CSF biomarkers in a cohort at risk for Alzheimer’s disease. Alzheimers Dement. (Amst.) 10, 188–195 (2018).
Roccati, E. et al. Modifiable dementia risk factors and AT(N) biomarkers: findings from the EPAD cohort. Front. Aging Neurosci. 16, 1346214 (2024).
Brown, B. M. et al. Self-reported physical activity is associated with tau burden measured by positron emission tomography. J. Alzheimers Dis. 63, 1299–1305 (2018).
Coomans, E. M. et al. Genetically identical twins show comparable tau PET load and spatial distribution. Brain 145, 3571–3581 (2022).
Vidoni, E. D. et al. Effect of aerobic exercise on amyloid accumulation in preclinical Alzheimer’s: a 1-year randomized controlled trial. PLoS ONE 16, e0244893 (2021).
Galle, S. A. et al. The effects of a moderate physical activity intervention on physical fitness and cognition in healthy elderly with low levels of physical activity: a randomized controlled trial. Alzheimers Res. Ther. 15, 12 (2023).
Schultz, S. A. et al. Cardiorespiratory fitness alters the influence of a polygenic risk score on biomarkers of AD. Neurology 88, 1650–1658 (2017).
Zlatar, Z. Z. et al. Dose-dependent association of accelerometer-measured physical activity and sedentary time with brain perfusion in aging. Exp. Gerontol. 125, 110679 (2019).
Iturria-Medina, Y., Sotero, R. C., Toussaint, P. J., Mateos-Pérez, J. M. & Evans, A. C. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat. Commun. 7, 11934 (2016).
Geffken, D. F. et al. Association between physical activity and markers of inflammation in a healthy elderly population. Am. J. Epidemiol. 153, 242–250 (2001).
Zarezadehmehrizi, A. et al. Exercise training ameliorates cognitive dysfunction in amyloid beta-injected rat model: possible mechanisms of Angiostatin/VEGF signaling. Metab. Brain Dis. 36, 2263–2271 (2021).
Pedrinolla, A. et al. Exercise training improves vascular function in patients with Alzheimer’s disease. Eur. J. Appl. Physiol. 120, 2233–2245 (2020).
Wrann, C. D. et al. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 18, 649–659 (2013).
Islam, M. R. et al. Exercise hormone irisin is a critical regulator of cognitive function. Nat. Metab. 3, 1058–1070 (2021).
Gorlovoy, P., Larionov, S., Pham, T. T. H. & Neumann, H. Accumulation of tau induced in neurites by microglial proinflammatory mediators. FASEB J. 23, 2502–2513 (2009).
Martin, L. et al. VEGF counteracts amyloid-β-induced synaptic dysfunction. Cell Rep. 35, 109121 (2021).
Yang, H.-S. et al. Plasma VEGFA and PGF impact longitudinal tau and cognition in preclinical Alzheimer’s disease. Brain 147, 2158–2168 (2024).
Rosa, A. D. et al. Long-term exercise training improves memory in middle-aged men and modulates peripheral levels of BDNF and Cathepsin B. Sci. Rep. 9, 3337 (2019).
Dicarlo, M. et al. Irisin levels in cerebrospinal fluid correlate with biomarkers and clinical dementia scores in Alzheimer disease. Ann. Neurol. 96, 61–73 (2024).
Folstein, M. F., Folstein, S. E. & McHugh, P. R. ‘Mini-Mental State’. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res 12, 189–198 (1975).
Wechsler, D. WMS-R: Wechsler Memory Scale-Revised (Psychological Corporation, 1987).
Holbrook, E. A., Barreira, T. V. & Kang, M. Validity and reliability of Omron pedometers for prescribed and self-paced walking. Med. Sci. Sports Exerc. 41, 670–674 (2009).
Tudor-Locke, C. et al. How many days of pedometer monitoring predict weekly physical activity in adults? Prev. Med. 40, 293–298 (2005).
Johnson, K. A. et al. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann. Neurol. 79, 110–119 (2016).
Rousset, O. G., Ma, Y. & Evans, A. C. Correction for partial volume effects in PET: principle and validation. J. Nucl. Med. 39, 904–911 (1998).
Papp, K. V., Rentz, D. M., Orlovsky, I., Sperling, R. A. & Mormino, E. C. Optimizing the preclinical Alzheimer’s cognitive composite with semantic processing: the PACC5. Alzheimers Dement. (N Y) 3, 668–677 (2017).
Wechsler, D. WAIS-R Manual: Wechsler Adult Intelligence Scale-Revised (Psychological Corporation, 1981).
Grober, E., Lipton, R. B., Hall, C. & Crystal, H. Memory impairment on free and cued selective reminding predicts dementia. Neurology 54, 827–832 (2000).
Monsch, A. U. et al. Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Arch. Neurol. 49, 1253–1258 (1992).
Stewart, A. L. et al. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med. Sci. Sports Exerc. 33, 1126–1141 (2001).
D’Agostino, R. B. et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 117, 743–753 (2008).
Tingley, D., Yamamoto, T., Hirose, K., Keele, L. & Imai, K. mediation: R Package for Causal Mediation Analysis. J. Stat. Softw. https://doi.org/10.18637/jss.v059.i05 (2014).
Acknowledgements
We thank the study participants of the Harvard Aging Brain Study. This work was supported by the National Institutes of Health (NIH) (grant numbers K23 AG084868 (W.-Y.W.Y.), K01 AG084816 (S.A.S.), P01 AG036694 (K.A.J. and R.A.S.), K24 AG035007 (R.A.S.), R01 AG062667 (J.P.C.) and R01 AG071865 (J.P.C.)); the Doris Duke Charitable Foundation Clinical Scientist Development Award (J.P.C.). This research was carried out in part at the Athinoula A. Martinos Center for Biomedical Imaging at the Massachusetts General Hospital, using resources provided by the Center for Functional Neuroimaging Technologies, P41EB015896, a P41 Biotechnology Resource Grant supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB), NIH. This work also involved the use of instrumentation supported by the NIH Shared Instrumentation Grant Program and/or High-End Instrumentation Grant Program, specifically, grant numbers S10RR021110, S10RR023401 and S10RR023043. A substantial amount of computational resources required for this research were performed on hardware generously provided by the Massachusetts Life Sciences Center (https://www.masslifesciences.com/). Funding sources had no involvement in study design, in the collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the article for publication.
Ethics declarations
Competing interests
The authors declare no competing interests relevant to the current study. Potential conflicts of interest outside the submitted work are included below. J.J.P. has served as a consultant for Eisai. K.A.J. has served as a consultant for Novartis, Merck and Janssen. R.A.S. has served as a consultant for AbbVie, AC Immune, Acumen, Alector, Apellis, Biohaven, Bristol Myers Squibb, Genentech, Ionis, Janssen, Oligomerix, Prothena, Roche and Vaxxinity over the past 3 years. K.A.J. and R.A.S. have received research funding from Eisai and Eli Lilly for public–private partnership clinical trials but do not have any personal financial relationship with the companies. J.P.C. has served as a consultant for ExpertConnect.
Peer review
Peer review information
Nature Medicine thanks Henry Brodaty, Susan Landau and Terry Therneau for their contribution to the peer review of this work. Primary Handling Editor: Jerome Staal in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Individual longitudinal Aβ, tau, PACC5 and CDR-SOB trajectories.
Individual trajectories of (a) global Aβ, (b) inferior temporal cortex (ITC) tau, (c) Preclinical Alzheimer’s Cognitive Composite-5 (PACC5) scores, and d) Clinical Dementia Rating Sum of Boxes (CDR-SOB) scores from all participants are shown without adjustment for any covariates. To allow clear visualization of the individual trajectories, participants were plotted according to high versus low baseline Aβ burden in columns and physical activity (mean steps per day) in rows, defined by above and below the median values. The trajectories are color coded by baseline Aβ burden according to the color bar, with each line representing one participant. The number of participants represented in each facet is provided for reference. The time of baseline PiB PET was used as the study baseline (time = 0). Timing of the first tau PET scan varied across participants (2.2 ± 1.5 years), as tau PET was introduced mid-study when it became available. Aβ = beta-amyloid; DVR = distribution volume ratio; PVC = partial volume correction; PiB = Pittsburgh compound-B; SUVR = standardized uptake value ratio.
Extended Data Fig. 2 Interactive association between baseline physical activity and Aβ burden on initial ITC tau burden.
Linear regression model revealed a significant interaction between baseline physical activity and Aβ burden on initial inferior temporal cortex (ITC) tau burden (Physical activity*Aβ: β = -0.19 [-0.30 to -0.08], p = 0.001), adjusting for age, sex, years of education, and time interval between study baseline and first tau scan. There was no significant independent effect of physical activity on tau (Physical activity: β = -0.06 [-0.18 to 0.05], p = 0.28). Statistical significance was assessed using two-tailed t-tests, with p < 0.05 considered statistically significant without adjustment for multiple comparisons. Physical activity (mean steps per day) was square-root transformed prior to model entry to account for skewness with improvement in model fit (reduced BIC by 2.2). Non-transformed mean steps per day was used to plot the model result to enhance interpretability. Baseline Aβ burden was modeled as a continuous variable. To visualize the model results, the estimated ITC tau burden across the range of baseline physical activity at representative levels of low versus high baseline Aβ burden are presented. Error bands represent the 95% confidence intervals for the estimated tau burden. Low and high Aβ are represented, for illustration purposes, by the mean Aβ burden of Aβ-negative (PiB PVC-DVR 1.17) and Aβ-positive (PiB PVC-DVR 1.85) participants respectively. Aβ = beta-amyloid; DVR = distribution volume ratio; ITC = inferior temporal cortex; PVC = partial volume correction; SUVR = standardized uptake value ratio.
Extended Data Fig. 3 Interactive association between cross-sectional Aβ and initial ITC tau burdens on baseline physical activity.
Linear regression model revealed no significant interaction between cross-sectional Aβ and ITC tau burdens on baseline physical activity (Aβ*ITC tau: β = -0.04 [-0.15 to -0.07], p = 0.44), adjusting for age, sex, years of education, and time interval between study baseline and first tau scan. There were further no significant independent effects of Aβ or ITC tau on baseline physical activity (Aβ: β = 0.10 [-0.07 to 0.28], t = 1.16, p = 0.25; ITC tau: β = 0.01 [-0.21 to 0.22], p = 0.96). Statistical significance was assessed using two-tailed t-tests, with p < 0.05 considered statistically significant without adjustment for multiple comparisons. Physical activity (mean steps per day) was square-root transformed prior to model entry, but non-transformed mean steps per day was used to plot the model result to enhance interpretability. Baseline Aβ burden was modeled as a continuous variable. To visualize the model results, the estimated means steps per day across the range of initial ITC tau burden at representative levels of low versus high baseline Aβ burden are presented. Error bands represent the 95% confidence intervals for the estimated mean steps per day. Low and high Aβ are represented, for illustration purposes, by the mean Aβ burden of Aβ-negative (PiB PVC-DVR 1.17) and Aβ-positive (PiB PVC-DVR 1.85) participants respectively. Aβ = beta-amyloid; DVR = distribution volume ratio; ITC = inferior temporal cortex; PVC = partial volume correction; SUVR = standardized uptake value ratio.
Extended Data Fig. 4 Association between physical activity levels and baseline Aβ burden on longitudinal (a) PACC5 decline and (b) CDR-SOB progression.
Linear mixed effects models revealed interaction between baseline physical activity levels (ordinal) and Aβ burden (continuous) on longitudinal PACC5 decline and CDR-SOB progression. Using the inactive subgroup as reference, all higher levels of physical activity were associated with slower Aβ-related PACC5 decline and CDR-SOB progression, except the slower CDR-SOB progression in the low activity group did not reach statistical significance (Table 3). To visualize the model results, the estimated trajectories based on representative levels of low versus high baseline Aβ burden across physical activity levels are presented, with error bands representing 95% confidence intervals for the estimated trajectories. Low and high Aβ are represented, for illustration purposes, by the mean Aβ burden of Aβ-negative (PiB PVC-DVR 1.17) and Aβ-positive (PiB PVC-DVR 1.85) participants respectively, defined using the conventional Aβ-positivity threshold (PiB PVC-DVR of 1.324). The horizontal dotted line represents thresholds for cognitive impairment (-1.5 PACC5 z-score) and functional decline (1.5 points on CDR-SOB). The vertical dot-dash line represents median duration of cognitive follow-up (9 years). Aβ = β-amyloid; DVR = distribution volume ratio; PiB = Pittsburgh compound-B; PVC = partial volume corrected; SUVR = standardized uptake value ratio.
Extended Data Fig. 5
Participant flow chart.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yau, WY.W., Kirn, D.R., Rabin, J.S. et al. Physical activity as a modifiable risk factor in preclinical Alzheimer’s disease. Nat Med (2025). https://doi.org/10.1038/s41591-025-03955-6
Received: 04 December 2024
Accepted: 12 August 2025
Published: 03 November 2025
Version of record: 03 November 2025
DOI: https://doi.org/10.1038/s41591-025-03955-6
.png)