Introduction
Human activities have increasingly exposed us to plastic fragments through the food chain, raising concerns about their impact on microbiota across various organisms. Microplastics (MP) and nanoplastics (NP), derived from the degradation of polystyrene (PS), polyethylene (PE), and polyvinyl chloride (PVC), have been shown to induce liver injury, hematopoietic damage, and testicular disorders in mammals by disrupting gut microbiota balance1,2,3. PS-MP and PE-MP exposure have been linked to gut barrier dysfunction, inflammation, and immune imbalances4,5. For instance, PE-MP exposure alters gut microbiota composition, favoring the overgrowth of pathogenic Staphylococcus aureus and promoting intestinal inflammation6,7. Similarly, PS-MP accumulation in mice is associated with colonic mucus dysfunction, intestinal barrier disruption, and increased abundance of pathogenic bacteria8. While the majority of research focuses on MP, the toxicity of micro-plastics is believed to increase with decreasing particle size9. However, the interaction between microscopic plastics, gut microbiota, and host is still a mystery, and their underlying mechanisms are poorly characterized. NP, due to their nanoscale size, can penetrate tissues and organs, posing potential biological hazards. However, the specific pathways through which NP disrupt gut microbiota balance and intestinal health are not well characterized. In this work, we investigate the biological accumulation of NP and their effects on the intestinal microenvironment, focusing on bacteria-host interactions.
Extracellular vesicles (EV), nanosized spherical structures encased in lipid bilayers, are released by both animal cells and bacteria and carry a diverse array of cargos, including proteins, DNA, RNAs, and lipids. These cargos are delivered to the extracellular environment for communication10,11,12,13. Recent studies have highlighted the role of EVs in intercellular communication, including their functions across species14. As the largest symbiotic ecosystem within the host, gut microbiota is key to maintaining intestinal homeostasis. The interaction between microbiota and the intestinal epithelium, often mediated by EVs, influences gut health and function15,16.
Given that both microbial and host-derived EVs regulate intestinal interactions, we hypothesize that NP may influence microbiota composition either directly or indirectly through these vesicular pathways. This study investigates the role of EV in host-microbiota interactions to elucidate whether NP directly disrupts intestinal microbiota composition or indirectly alters it by influencing host cellular processes, thereby uncovering the mechanisms underlying NP-induced changes in gut microbiota and intestinal environments.
Results and discussion
Distribution of nanoplastics and their impact on the intestinal environment
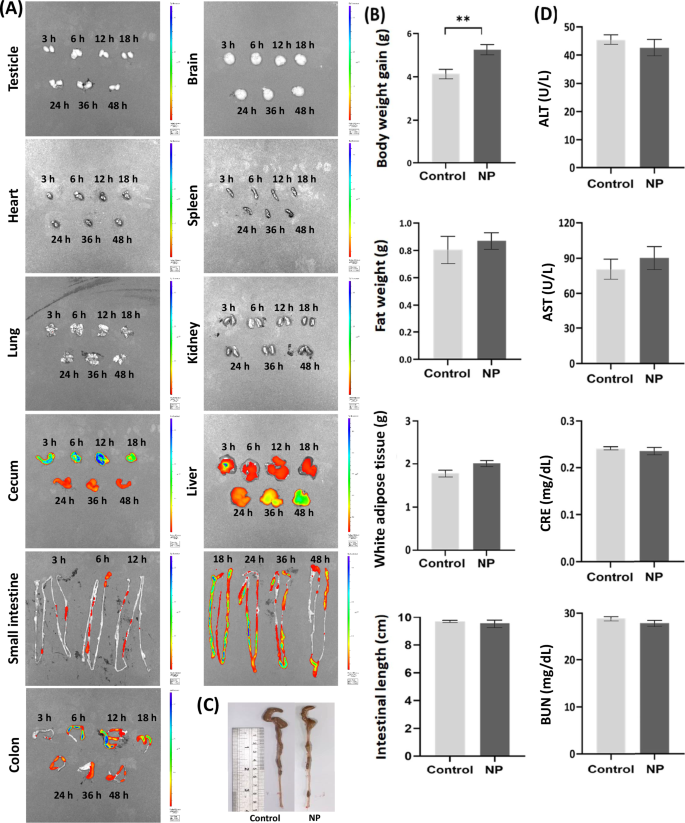
Mice administered fluorescently (FluoSpheres carboxylate-modified microspheres) labeled NP (100 nm) displayed substantial NP accumulation in the cecum, liver, small intestine, and colon at 3, 6, 12, 18, 24, 36, and 48 h post-administration (Fig. 1A). These findings align with previous reports of NP persistence in the gut17. Given that NP can persist in the colon for up to 48 h, this study further examined their effects on the intestinal environment through oral administration of polystyrene (100 nm; 2 × 1011 particles/mouse, concentration determined by Nanosight) four times a week (on days 1, 3, 5, and 7 of each week). Recent studies have indicated that polyvinyl chloride-MP may induce hepatic injury1, while PS-MP exposure has been linked to disruptions in blood triglyceride metabolism17. Additionally, PS-MP has been associated with obesity and increased adiposity in mice18. In this study, prolonged NP exposure through oral administration (four times per week for 12 weeks) resulted in an increased body weight in NP-treated mice compared to controls (5.17 g vs. 4.03 g; p > 0.01), although no significant changes in white adipose tissue weight or liver weight were observed (Fig. 1B). The length of the intestine can be used as an indicator to assess the severity of intestinal inflammation19. Previous studies have confirmed that both MP and NP pose a threat to the intestinal environment and microbiota1,2,3,4,5,6,7,8. The absence of intestinal shortening in NP-exposed mice (Fig. 1C) suggests that intestinal bacteria, rather than inflammation, may be the primary target of NP-induced effects. Despite the established toxicity of MP on liver and kidney functions1,17, NP exposure for 12 weeks did not significantly alter serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine (CRE), or blood urea nitrogen (BUN) levels (Fig. 1D). This indicates that the intestinal microbiota and barrier may be more directly affected by NP.
A Distribution of NP (100 nm) in mice observed via in vivo imaging system (IVIS) for measuring fluorescence intensity (Near-infrared fluorescence). Effects of NP on (B) body weight gain, fat weight, and white adipose tissue. C intestinal length, and (D) serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine (CRE), and blood urea nitrogen (BUN) in mice orally administered with NP for 12 weeks. Data were shown as mean ± SEM (n = 12).
Nanoplastics influence host intestinal microRNA to regulate the microenvironment and barrier function
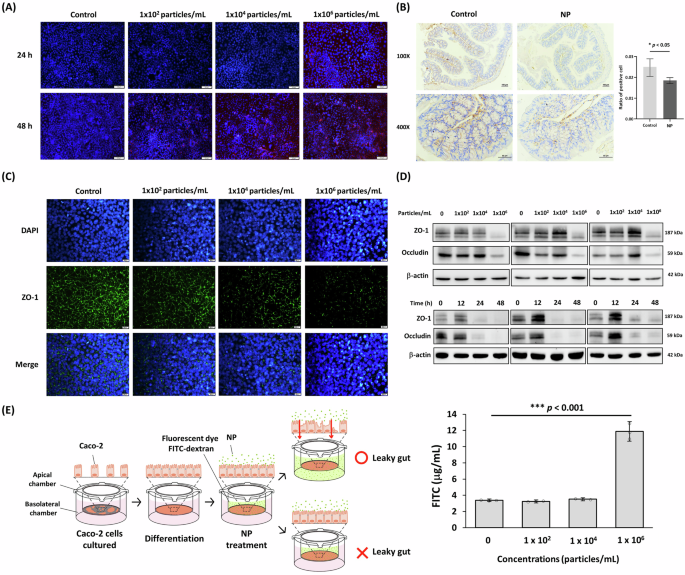
Since the intestine is the primary tissue affected by MP/NP, we further examined the impact of NP on enterocytes and found that NP (FluoSpheres nano-polystyrene; 104 and 106 particles/mL) entered enterocyte-like differentiated Caco-2 cells after 24 and 48 h of treatment (Fig. 2A). In both the mouse intestine and enterocyte-like differentiated Caco-2 cells, NP exposure reduced the expression of tight junction proteins, including zonula occludens-1 (ZO-1) (Fig. 2B, C) and occludins (OCC) (Fig. 2D). This disruption led to characteristic intestinal damage, resulting in increased intestinal permeability (leaky gut) (Fig. 2E). Although recent studies have demonstrated that NP and MP significantly impair gut barrier function4,20, the underlying mechanisms remain to be elucidated.
A Accumulation of NP in enterocyte-like differentiated Caco-2 cells after treatment for 24 and 48 h. Red stain: FluoSpheres nano-polystyrene (NP); Blue stain: DAPI. Scale bars indicate 100 µm. B Intestinal ZO-1 level in NP-treated mice for 12 weeks via IHC stain. Scale bars indicate 100 µm (upper) and 50 µm (lower). Data were shown as mean ± SEM (n = 12) (* p value < 0.05). C Enterocyte-like differentiated Caco-2 cells were treated with different concentrations of NP (102, 104, 106 particles/mL) for 48 h. Immunofluorescence staining of tight junction ZO-1 (green). Cell nuclei were counterstained by DAPI (blue). Scale bars indicate 20 µm. D The suppressions of ZO-1 and occludin in enterocyte-like differentiated Caco-2 cells with NP treatment measured by Western blot for concentration-dependent manner (upper) and time-dependent manner (below). E Intestinal leakage evaluation in enterocyte-like differentiated Caco-2 cells treated with NP for 48 h by measuring FITC-dextran permeability. Data were shown as mean ± SD (n = 3).
Transcriptome analysis was performed in this study to evaluate the impact of NP on host intestinal function. The volcano plot of differentially expressed genes illustrates individual genes, with significant up-regulated and down-regulated genes (Log2(FC) > 2 and p-value < 0.05) highlighted in green and red, respectively (Supplementary Fig. 1A). A Heatmap displayed the differentially expressed protein-coding genes that were up-regulated by NP treatment, with criteria set at a twofold expression change and p-value < 0.05, along with Gene ontology (GO) functional enrichment (Supplementary Fig. 1B). Similarly, a heatmap showed the protein-coding genes down-regulated by NP treatment under the same criteria, and GO analysis revealed that NP exposure significantly affected intestinal gene expression and metabolic functions in mice (Supplementary Fig. 1C).
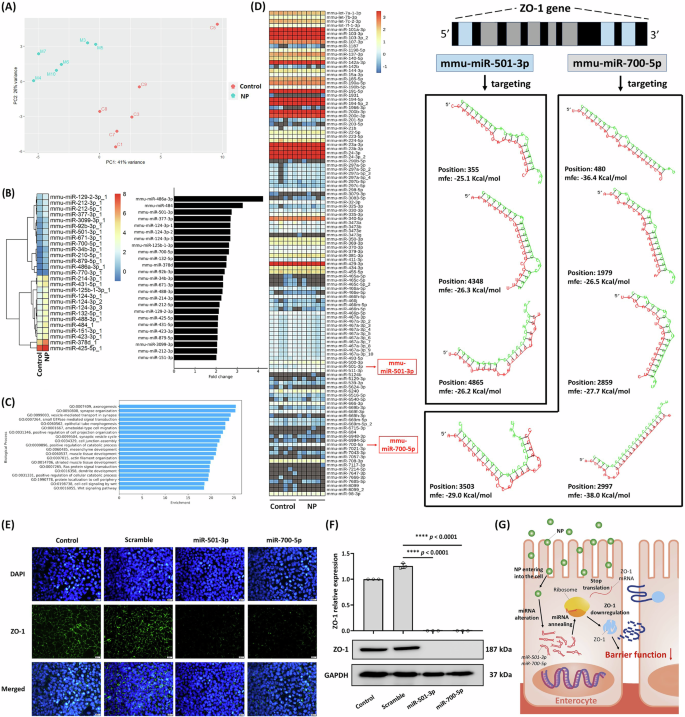
Gene expression is often regulated by microRNAs (miRNAs), short non-coding RNAs typically 18-23 nucleotides in length. To explore the impact of NP on the intestinal host, we examined miRNAs and their regulatory roles. Although previous studies have quantified RNA in human stool and identified miRNAs as potential indicators of the intestinal environment21,22, the presence and function of fecal miRNAs in the normal gut remain largely unexplored. In this study, we identified intestinal miRNAs and investigated their role in shaping gut microbiota composition. Principal component analysis (PCA) of miRNA diversity in mouse feces revealed that NP treatment significantly altered miRNA profiles and reduced miRNA diversity (Fig. 3A). The specific miRNAs that were significantly upregulated by NP exposure are shown in Fig. 3B. Further analysis demonstrated that these miRNAs regulate key physiological functions, including those related to intestinal cell junctions (GO: 0034329) (Fig. 3C).
A Principal components analysis (PCA) of miRNA sequencing results from colonic tissue of NP-treated mice (n = 6) for 12 weeks. B Heatmap and fold change of significantly different miRNAs post NP treatment (p < 0.05). C Gene Ontology (GO) annotations predicting gene expression changes following miRNA interference (GO0034329 for cell junction) in the colon. D Heatmap predicting miRNAs (miR-501-3p and miR-700-5p) potentially interfering with ZO-1. Validation of miR-501-3p and miR-700-5p interference with ZO-1 expression in enterocyte-like differentiated Caco-2 cells by (E) Immunofluorescence stain (Scale bars indicate 20 µm) and (F) Western blot. Data are presented as means ± SD (n = 3). G Schematic illustration of NP impact on ZO-1 expression and intestinal barrier function.
This study further investigated the miRNAs significantly upregulated by NP treatment, specifically those regulating the expression of the ZO-1 gene. As shown in Fig. 3B, two miRNAs, mmu-miR-501-3p (2.72 fold, p < 0.001) and mmu-miR-700-5p (2.6 fold, p < 0.001), were markedly increased following NP exposure. These miRNAs have the potential to interfere with ZO-1 expression. Predictive analysis revealed that mmu-miR-700-5p targets multiple sites within the ZO-1 gene (positions 480, 1979, 2859, 2997, and 3503), while mmu-miR-501-3p targets positions 355, 4348, and 4865, thereby suppressing ZO-1 expression in the intestine (Fig. 3D). Furthermore, transfection with synthetic mmu-miR-501-3p and mmu-miR-700-5p similarly inhibited ZO-1 expression in enterocyte-like differentiated Caco-2 cells (Fig. 3E, F). While previous studies have shown that PS-MP exposure reduces tight junction protein expression in mice4,23, these studies did not explore the underlying mechanisms. Our findings demonstrate that NP may interfere with tight junction protein expression by modulating miRNAs in intestinal cells, thereby disrupting the intestinal environment (Fig. 3G).
Similar results were observed in cell experiments. The miRNA content of EVs derived from goblet cell-like LS174T cells was altered following NP treatment (Supplementary Fig. 2). GO analysis revealed that these altered miRNAs regulate cell junction-related pathways. Predictive analysis indicated that the upregulation of has-miR-98-3p, has-miR-548z, has-miR-548h-3p, has-miR-548d-3p, has-miR-548az-5p, has-miR-12136, and has-miR-101-3p caused by NP treatment has the potential to specifically interfere with the ZO-1 gene (Supplementary Fig. 3).
The risk of nanoplastics interfering with intestinal mucin expression
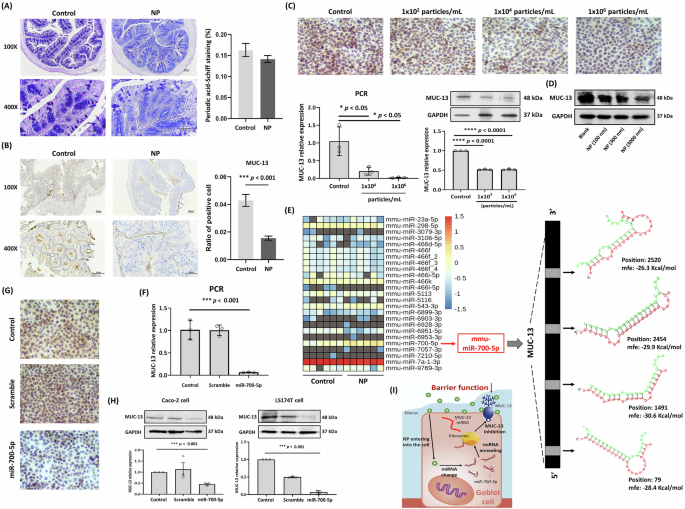
Mucin (MUC), primarily secreted by goblet cells and enterocytes in the gut, plays a crucial role in maintaining intestinal barrier integrity. High levels of MUC are typically observed in the colon24,25,26. Previous studies have demonstrated that exposure to PS or PE reduces MUC levels in the gut7,27. The Alcian blue-Periodic acid Schiff (AB-PAS) stain is widely used in histology to detect carbohydrates in tissues. Periodic acid oxidizes hydroxyl groups on adjacent carbon atoms in carbohydrate molecules, forming aldehyde groups that react with Schiff’s reagent to produce a magenta or purple-red coloration. In this study, the MUC index, as indicated by carbohydrate levels, was reduced in mice following 12 weeks of NP treatment, although this decrease was not statistically significant (Fig. 4A). However, NP treatment significantly reduced the expression of MUC-13 (but not MUC-5B) in mice (Fig. 4B), as well as in enterocyte-like differentiated Caco-2 cells, as shown by immunocytochemistry (ICC), qPCR, and Western blot (Fig. 4C). Similar reductions were observed in goblet-like LS174T cells, confirmed through Western blot analysis (Fig. 4D).
A Alcian blue-periodic acid Schiff (AB-PAS) staining of intestinal mucus in mice with or without NP treatment. Scale bars indicate 100 µm (upper) and 50 µm (lower). Data were shown as mean ± SEM (n = 12). B IHC stain of intestinal MUC-13 in NP-treated mice. Scale bars indicate 100 µm (upper) and 50 µm (lower). Data were shown as mean ± SEM (n = 12) (*** p value < 0.001). C MUC13 expression in NP-treated enterocyte-like differentiated Caco-2 cells for 48 h by ICC stain (Scale bars indicate 20 µm), qPCR, and Western blot. Data are presented as means ± SD (n = 3). Significant difference was shown by different letters (* p < 0.05; *** p < 0.001). D Western blot analysis of MUC-13 levels in goblet-like LS174T cells treated with NP for 48 h. E Heatmap predicting various intestinal miRNAs suppressing MUC-13 in NP-exposed mice. Validation of miR-700-5p interference on MUC-13 in enterocyte-like differentiated Caco-2 cells by (F) qPCR. G ICC stain (Scale bars indicate 20 µm), and (H) Western blot. Data are presented as means ± SD (n = 3). Significant difference was shown by different letters (***p < 0.001). I Schematic of NP impact on MUC-13 and mucus secretion in the gut.
It has been suggested that MUC-13 protects against dextran sulfate sodium-induced colitis by inhibiting enterocyte apoptosis28. Our findings indicate that NP plays a significant role in intestinal dysfunction by reducing MUC-13 levels; however, the mechanisms through which NP regulates MUC-13 expression remain to be elucidated. To investigate this, we analyzed intestinal miRNAs and their relationship with MUC-13 expression using RNA sequencing. A heatmap of all intestinal miRNAs with potential binding sites on the MUC-13 gene was constructed (Fig. 4E). Among these, mmu-miR-700-5p was the only miRNA significantly upregulated by NP treatment (2.6-fold, p < 0.001). Predictive analysis of miRNA interference positions on the MUC-13 gene, using hybrid prediction techniques, revealed that mmu-miR-700-5p targets specific sites on the MUC-13 gene (positions 79, 1491, 2454, and 2520), leading to a reduction in MUC-13 expression. In addition, transfection of synthetic mmu-miR-700-5p into enterocyte-like differentiated Caco-2 cells significantly suppressed MUC-13 expression, as demonstrated by qPCR (Fig. 4F) and ICC (Fig. 4G). Furthermore, treatment of both enterocyte-like differentiated Caco-2 cells and goblet cell-like LS174T cells with mmu-miR-700-5p confirmed its inhibitory effect on MUC-13 expression, as evidenced by Western blot analysis (Fig. 4H). A schematic illustration depicting the proposed mechanism by which NP reduces MUC-13 expression in the gut is provided (Fig. 4I).
Several hundred miRNAs have been identified in exosomes and extracellular vesicles (EVs) derived from feces and edible plants, with their profiles analyzed for their potential to target and regulate genes in various hosts29,30,31. Beyond miRNAs, EVs derived from intestinal cells, the composition of mucus within the intestinal environment, and interactions among microbial communities are critical factors influencing intestinal microbiota composition and overall host health29,30,31,32,33. While numerous studies have shown that nanoplastics (NP) and microplastics (MP) contribute to gut microbiota imbalance, the mechanisms driving these disruptions remain poorly understood1,2,3,4,5,6,7,8.
Nanoplastics directly and indirectly induce imbalance in gut microbiota
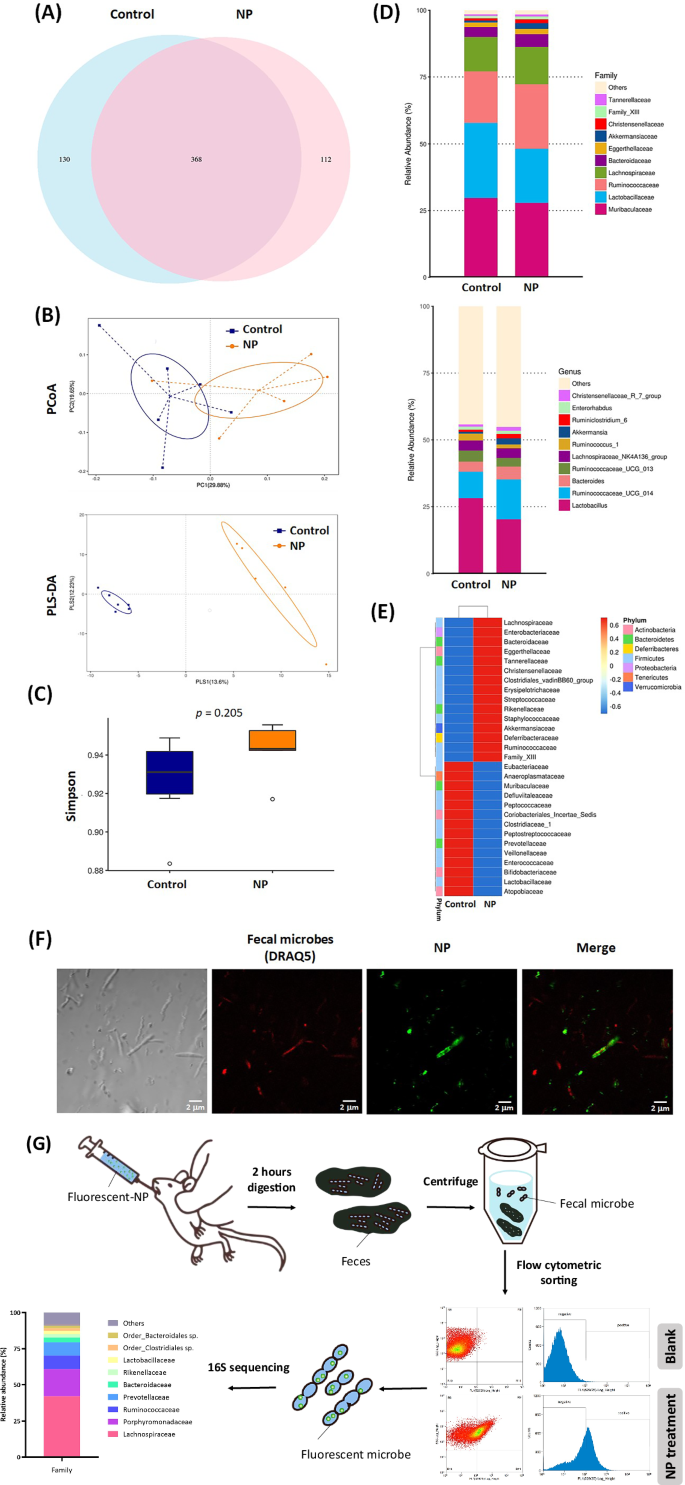
Compared to the control group, the NP treatment group displayed an increase of 112 unique bacterial species after 12 weeks of treatment, as shown in the Venn diagram (Fig. 5A). At earlier time points, 171 and 161 unique bacterial species were identified after 4 weeks (Supplementary Fig. 4) and 8 weeks (Supplementary Fig. 5) of NP treatment, respectively. While the number of unique bacterial species decreased with prolonged NP exposure, the top 10 bacterial families shared between the NP treatment and control groups-including Ruminococcaceae, Lactobacillaceae, and Lachnospiraceae-did not show significant changes in abundance. Interestingly, Akkermansia, a next-generation probiotic bacterium, exhibited a higher abundance in the NP treatment group compared to the control group, particularly from 8 weeks to 12 weeks of treatment. While the underlying mechanisms remain unclear, these observations suggest that NP treatment may exert a targeted and systematic effect on specific bacterial species (Supplementary Fig. 5).
A Venn diagram showing shared and unique ASVs of gut microbiota between the control and NP treatment groups. B β-diversity analysis of gut microbiota samples using principal coordinate analysis (PCoA) and Partial Least Squares Discriminant Analysis (PLS-DA). C α-diversity analysis of microbiota composition based on the Simpson index. The center line represents the median (50th percentile). The box represents the interquartile range (IQR) between the 25th percentile (Q1) and the 75th percentile (Q3). Whiskers extend to the smallest and largest values within 1.5 × IQR. Outliers are shown as individual points. Group Control: Min = 0.8835, Q1 = 0.9197, Median = 0.9311, Q3 = 0.9418, Max = 0.9489. Group NP: Min = 0.9170, Q1 = 0.9424, Median = 0.9428, Q3 = 0.9503, Max = 0.9557. D Relative abundance of the top 10 classifications for family and genus levels of gut microbiota. E Heatmap of the top 35 species at the family level. Group information is presented vertically, while species annotation is displayed horizontally. F Fluorescence microscopy showing NP uptake by fecal microbes in mice 6 h after administration. Bacterial DNA is stained with DRAQ5 (red), and fluoresbrite YG carboxylate microspheres represent NP (yellow-green). Scale bars indicate 2 µm. G Identification of gut microbes that have taken up NP using flow cytometric sorting and 16S rRNA sequencing. α-Diversity (Simpson index) was calculated using QIIME2, with group differences assessed using t-tests and Wilcoxon tests. β-Diversity was analyzed using UniFrac distance metrics and visualized through PCoA and PLS-DA plots. Statistical significance of β-diversity differences was assessed using t-tests and Wilcoxon tests. Taxa differences were analyzed using Welch’s t-test in STAMP and permutation tests in R’s metagenomeSeq package, with p-values adjusted using the Benjamini and Hochberg False Discovery Rate method. Data are presented as means ± SD (n = 6).
Additionally, beta diversity analysis of intestinal microbiota was conducted using Principal Co-ordinates Analysis (PCoA) and Partial Least Squares Discriminant Analysis (PLS-DA) (Fig. 5B), while alpha diversity was assessed using the Simpson index (Fig. 5C). The results demonstrated a high level of dispersion in the beta diversity of gut microbiota between the NP treatment group and the control group. Further examination of the top 10 relatively abundant bacterial families revealed that NP treatment decreased the relative abundance of Lactobacillaceae and increased that of Ruminococcaceae (Family level). At the genus level, Lactobacillus was identified as the primary microorganism that decreased, whereas Ruminococcaceae-UCG-014 was the main bacterium that increased in abundance (Fig. 5D). Figure 5E provides a detailed analysis of the indicator variation in intestinal microbiota caused by 12 weeks of NP treatment, highlighting specific shifts in microbial composition.
To investigate the direct impact of NP on the intestinal microbiota, mice were orally administered Fluoresbrite YG carboxylate-modified microspheres (100 nm, yellow-green fluorescent). After 2 h, fecal bacteria were collected and labeled with DRAQ5 dye (red fluorescent) for analysis. Confocal microscopy revealed NP accumulation in fecal bacteria, indicating that NP was taken up by these microorganisms (Fig. 5F). To further elucidate the changes in microbiota caused by NP and identify key bacterial taxa affected, bacteria carrying NP-labeled fluorescence were sorted using flow cytometry, followed by 16S rRNA sequencing to analyze bacterial species. The results showed that NP predominantly entered Lachnospiraceae (42.0%), followed by Porphyromonadaceae (18.9%) and Ruminococcaceae (9.3%) (Fig. 5G).
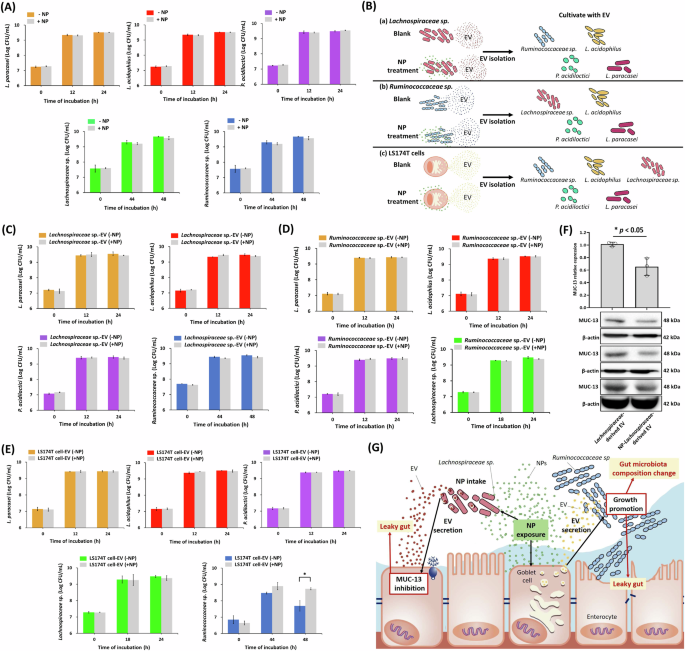
Due to the reduction of Lactobacillus and the increased proportion of Ruminococcaceae in the intestines caused by NP (Fig. 5D), and given that Lachnospiraceae are the primary intestinal bacteria consuming NP, the mechanism underlying these microbial changes are of particular interest in this study. To investigate the mechanisms of NP-induced alterations in the intestinal microbiota, we evaluated the effects of NP on the growth of key intestinal bacteria, including L. paracasei (BCRC 14023), L. acidophilus (BCRC 14079), P. acidilactici, Lachnospiraceae sp. (ATCC-TSD-26), and Ruminococcaceae sp. (ATCC-TSD-27). As shown in Fig. 6A, NP treatment did not affect the growth of these microorganisms. Additionally, we assessed the non-toxic effects of NP on the growth of lactic acid bacteria, including L. helveticus, L. amylovorus, L. paracasei, L. delbrueckii, and L. fermentum (Supplementary Fig. 5). These findings suggest that the effects of NP on the intestinal microbiota are not directly caused by NP toxicity but may instead occur through other mechanisms.
A The effects of NP treatment (1 × 1010 particles/mL) on the growth of various lactic acid bacteria (L. paracasei, L. acidophilus, and P. acidiloctici), Lachnospiraceae sp. (TSD-26; ATCC), and Ruminococcaceae sp. (TSD-27; ATCC). B Schematic of experimental process by interactions between bacterial EV and cell-derived EV. C Impact of Lachnospiraceae sp.-derived EV without or with NP treatment (1 × 1010 particles/mL) for 18 h on the growth of different bacterial species (L. paracasei, L. acidophilus, P. acidiloctici, and Ruminococcaceae sp.). D The impact of Ruminococcaceae sp.-derived EV without or with NP treatment (1 × 1010 particles/mL) for 44 h on the growth of different bacterial species (L. paracasei, L. acidophilus, P. acidiloctici, and Lachnospiraceae sp.). E Impact of goblet-like LS174T cells without or with NP treatment (106 particles/mL) for 48 h on the growth of different bacterial species (L. paracasei, L. acidophilus, P. acidiloctici, and Lachnospiraceae sp. and Ruminococcaceae sp.). F Western blot of MUC13 inhibition by Lachnospiraceae sp.-derived EV. Data were shown as mean ± SD (n = 3) (* p value < 0.05). G Schematic representation summarizing the proposed mechanisms of NP-induced modulation of gut microbiota via EV. NP are taken up by Lachnospiraceae, whose EV suppress MUC13 expression in goblet cells. Concurrently, NP-modified EV from goblet cells promote the growth of Ruminococcaceae, collectively contributing to gut microbiota imbalance and potential intestinal barrier dysfunction.
In many studies, EV have been identified as primary messengers facilitating communication between intestinal microorganisms and the host. Host-secreted EV can selectively regulate intestinal microbiota, while interactions among microorganisms (bacteria-bacteria interactions) are also mediated by EV, influencing processes such as biofilm formation, antibacterial effects, and microbial survival34,35,36. Lachnospiraceae, a bacterial family present in the intestine, is known for its ability to degrade mucin and produce antibacterial short-chain fatty acids. It has long been recognized as playing a crucial role in maintaining the ecological balance of the intestinal environment37,38.
Flow cytometry screening and microbial sequencing revealed that Lachnospiraceae were the dominant bacterial family taking up NP. Consequently, the role of NP entry into Lachnospiraceae in influencing the intestinal environment became the next focus of investigation. This study aimed to explore the indirect effects of NP on gut microbiota mediated by Lachnospiraceae as outlined in the experimental design (Fig. 6B). First, Lachnospiraceae sp. was cultured with or without NP treatment. The EV derived from Lachnospiraceae sp. in the culture medium were then collected. Finally, the effects of these Lachnospiraceae sp.-derived EV on the growth of various intestinal microorganisms (L. paracasei, L. acidophilus, P. acidilactici, and Ruminococcaceae sp.) were evaluated. The results showed that Lachnospiraceae sp.-derived EV did not influence the growth of intestinal bacteria (Fig. 6C). Since Ruminococcaceae were the predominant microorganism that increased following 12 weeks of NP treatment in mice, this study also examined the impact of Ruminococcaceae sp.-derived EV on intestinal bacteria. Similar to the findings for Lachnospiraceae sp.-derived EV, Ruminococcaceae sp.-derived EV did not affect the growth of intestinal bacteria (Fig. 6D).
Taken together, we hypothesize that the imbalance in intestinal microbiota caused by NP is not a result of bacteria-bacteria interactions but rather a consequence of NP-induced dysregulation in the host intestine, which subsequently affects the microbiota. This hypothesis is supported by previous studies suggesting that certain miRNAs present in EV can regulate the growth of specific microorganisms. For instance, miR-1226-5p derived from intestinal EV has been shown to enhance the growth of Escherichia coli, while miR-515-5p promotes the growth of Fusobacterium nucleatum in mice. The differential ability of specific host-derived miRNAs to enter bacteria may partly explain their distinct effects on bacterial gene transcription and growth29. Additionally, another study indicated that fecal miRNA transplantation could have therapeutic potential by restoring gut microbiota composition39. These findings align with and support the hypothesis proposed in the present study.
To confirm that NP primarily affects intestinal-derived EV, which indirectly lead to changes in the intestinal microbiota, this study utilized goblet-like LS174T cells with or without NP treatment for investigation (Fig. 6B). The results demonstrated that while NP treatment did not alter EV size, it significantly increased EV production by goblet-like LS174T cells (Supplementary Fig. 6). Furthermore, EV derived from NP-treated goblet-like LS174T cells significantly enhanced the growth and survival of Ruminococcaceae sp. (Fig. 6E). In contrast, EV derived from enterocyte-like Caco-2 cells, regardless of NP treatment, did not exhibit a similar growth-promoting effect on Ruminococcaceae sp. (Supplementary Fig. 7). These findings suggest that goblet-like cells play a more critical role than enterocyte-like cells in regulating the gut microbiome. Consequently, NP interference with goblet-like cell function indirectly affects the growth of Ruminococcaceae sp., as shown in Figs. 4, 5B, and 6E.
Ruminococcaceae sp. is a significant bacterial species in the gut. Research on gastrointestinal dysfunction has indicated that an abnormal increase in Ruminococcaceae sp. abundance on the intestinal mucosa is associated with Autism Spectrum Disorder (ASD)40. Additionally, studies have demonstrated that young mice exposed Ruminococcaceae sp. bacteria and their metabolites exhibited adverse effects on nerve damage and aging41. Furthermore, Ruminococcaceae sp. has been implicated in the progression of liver fibrosis42. In summary, we hypothesize that NP promotes the growth of Ruminococcaceae sp. by modulating goblet-like cell function, which could represent a potential risk factor for NP exposure in the host.
The interaction between the mucus layer and the microbiota plays a critical role in stabilizing the intestinal environment in the host. Beyond serving as a scaffold for intestinal microbiota, the mucus layer also provides a nutrient source (carbon) for bacterial growth. However, abnormalities in the mucus layer, dysbiosis, and disrupted mucus-microbiota interactions can potentially lead to intestinal diseases43,44. To explore the effects of Ruminococcaceae sp. on MUC-13 expression, we evaluated the impact of Ruminococcaceae sp.-derived EV, with or without NP treatment, on goblet-like LS174T cells. The results showed that Ruminococcaceae sp.-derived EV did not significantly alter MUC-13 expression (Supplementary Fig. 8). In contrast, NP-treated Lachnospiraceae sp.-derived EV significantly inhibited MUC-13 expression in goblet-like LS174T cells (Fig. 6F). As shown in Fig. 5G, NP not only extensively enters and accumulates in Lachnospiraceae, but also results in Lachnospiraceae sp.-derived EV suppressing MUC-13 levels through the regulation of host microRNAs (Fig. 4).
Exposure to polystyrene-NP/MP (0.5 and 50 μm) has been reported to decrease the abundance of Oscillospira and Anaerostipes while increasing the abundance of Parabacteroides, Prevotella, Dehalobacterium, Ruminococcus, Bilophila, Adlercreutzia, Plesiomonas, Halomonas, and Acinetobacter45. Additionally, exposure to microplastics has been shown to reduce mucus secretion and induce inflammation4,46. While the risks of NP exposure on the gut have been documented, the underlying mechanisms and their impact on the gut microbiome remain poorly understood47. Notably, a single miRNA may target hundreds of bacterial mRNAs, further complicating the interplay between host miRNAs and the gut microbiota48.
Based on the aforementioned findings, it can be concluded that PS-NP treatment increases EV concentrations in both the host and intestinal microbiota. These EVs, particularly those derived from goblet-like LS174T cells, promote the proliferation of Ruminococcaceae sp., thereby contributing to gut microbiota imbalance. To further validate the impact of NP on EV levels in the gut lumen, we quantitatively measured and confirmed EV concentrations in culture media derived from mouse intestinal tissue and fecal microbiota cultures, with or without NP treatment (Supplementary Fig. 9). Our results indicate that NP modifies the gut microbiota both directly and indirectly, while also disrupting host intestinal functions, ultimately leading to a deteriorated intestinal environment. These findings suggest a mechanism by which NP affects both the microbiota and the intestinal environment (Supplementary Fig. 10). Specifically, NP treatment alters the miRNA profile of intestinal cells, disrupts gut permeability, modifies MUC expression, and causes gut microbiota imbalance. These insights provide valuable tools for assessing the risks posed by plastic debris to human and environmental health.
The risks posed by nanoscale plastic particles remain poorly understood. This study utilized an omics-based approach combined with host-bacteria interaction analyses to elucidate the mechanisms by which polystyrene NP induce changes in the microbiota and deterioration of the intestinal environment. Polystyrene NP significantly impact the gut microenvironment through complex interactions involving both host-derived and bacterial-derived EV. By altering the expression of miRNAs and proteins associated with the intestinal barrier, NP contribute to gut microbiota dysregulation.
Unlike most previous studies that focused on MP and their effects on gut microbiota, this study used NP to investigate their distinct impacts. Although NP did not cause broad shifts in the overall gut microbiota composition, they specifically influenced certain bacterial taxa, such as Lachnospiraceae and Ruminococcaceae. The mechanisms underlying these changes were clarified, revealing that NP-mediated host-microbiota interactions occur through EV. Notably, NP were primarily ingested by Lachnospiraceae, whose EV suppressed mucin-13 expression. Simultaneously, NP modified the EV released by intestinal goblet cells, promoting an increase in Ruminococcaceae abundance (Fig. 6G). These findings highlight an indirect pathway through which NP disrupt gut health, emphasizing the potential long-term risks associated with environmental NP pollution. This study also underscores the critical role of host-microbiota interactions mediated by EV, offering valuable insights into the risks of plastic debris and providing a foundation for future research into its impact on human and environmental health.
Methods
Quantitative analysis of NP
NP (1 mL; 100 nm; Sigma Aldrich, St. Louis, MO, USA) was used for nanoparticle tracking analysis (NTA) (NanoSight LM10-HS, Malvern Instruments, Malvern, UK) to confirm the number and particle size of NP.
NP distribution and animals
The C57BL/6 male mice (6-week-old; purchased from the Laboratory Animal Center of National Cheng Kung University) were given nonfluorescent 57W5 feed (TestDiet, Saint Louis, MO, USA) and NP (100 nm, FluoSpheres carboxylate-modified microspheres) (Invitrogen, Eugene, OR, USA) with labeled far-infrared fluorescence (Ex/Em: 715/755 nm). The organs were collected after sacrification, and far-infrared fluorescent dye was excited to generate signals for observe NP accumulation using IVIS Spectrum imaging system. The C57BL/6 male (6-week-old) were keeped in a 12 h/12 h light/dark cycle at 22 ± 2 °C (approval No. 108247 and No. 109330). The mice were randomly assigned to control and NP-treated group (n = 6). The NP (100 nm, 0.2 mL, 2 × 1011 particles/mL) (Sigma Aldrich, St. Louis, MO, USA) was orally administered to the mice for 12 weeks (four times/week). The colon, feces, and serum were collected after sacrification. The colon tissues were immersed in a 4% formaldehyde solution for fixation for hematoxylin/eosin and immunohistochemistry staining.
Serum biochemical analysis
Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine (CRE), and blood urea nitrogen (BUN) levels were measured according to the guideline of commercial kits (Randox Laboratories Ltd, Antrim, UK).
Observation of cellular uptake of NP
The Caco-2 cells were seeded (105 cells/cm2) and induced differentiation49. The enterocyte-like differentiated Caco-2 cells were treated with NP (100 nm, Fluoresbrite YG carboxylate microspheres) (Polysciences, Warrington, PA, USA). Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (Santa Cruz, Dallas, Texas, USA), and intracellular accumulation of NP was observed using a fluorescence microscope (FV3000, Olympus, Shinjuku City, Japan).
Immunofluorescence, immunocytochemistry, alcian blue-periodic acid Schiff (AB-PAS) stain
The ZO-1 (ab221547, Abcam, Bristol, UK) expression in enterocyte-like differentiated Caco-2 cells and mice treated with NP was observed by immunofluorescence staining and immunocytochemistry staining. MUC13 (bs-10074R, Bioss, Boston, Massachusetts, USA) expression in enterocyte-like differentiated Caco-2 cells, goblet-like LS174T cells, and mice was evaluated by immunocytochemistry staining. The amount of mucus in colon tissue was determined using AB-PAS staining (Abcam, Bristol, UK).
In vitro cellular permeability
Permeability of differentiated Caco-2 cell monolayers was evaluated by FITC-dextran efflux assay. The Caco-2 cells (2 × 104 cells/cm2) were seeded in Transwell® plates (Corning; USA), and the culture medium was added to the apical and basal chambers for 21 days. After NP induction, cell monolayer was washed by PBS, and FITC-dextran solution (Sigma Aldrich, USA) was added. The FITC-dextran permeability was measured (excitation: 480 nm and emission: 525 nm) after treatment for 2 h50.
Real-time PCR and western blot
Total RNA was extracted from renal tissues and cells using TRIzol® (Ambion, Waltham, MA, USA) following the manufacturer’s instructions. Complementary DNA (cDNA) was synthesized from 2 μg of total RNA using the MMLV Reverse Transcription Kit (Protech, Taipei, Taiwan). Quantitative PCR (qPCR) was performed using the Fast SYBR™ Green Master Mix (4385612, Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer’s protocol. mRNA expression levels were analyzed using real-time qPCR (StepOne Real-Time PCR System, USA). MUC13 primers: forward 5′-AGAAACATTCCATGGCCTATCAA-3′, reverse 5′-TGTCCATAAACAGATGTGCCAAA-3′; GAPDH primers: forward 5′-TGACGTGCCGCCTGGAGAAA-3′, reverse 5′-AGTGTAGCCCAAGATGCCCTT-3′. The relative expression of target gene mRNA was normalized to GAPDH and calculated using the 2−ΔΔCt method. For RNA interference (RNAi), cells (5 × 105 cells per well) cultured in 6-well plates were transfected using the TransIT-X2® Dynamic Delivery System (MiRus Bio, Inc., Madison, WI, USA). miRNA was used solely for transient transfection. siRNA (25 nM) was applied to knock down target gene expression in the cells. Sequence of scrambled control: ACACACAACACUGUCACAUUCCA, mmu-miR-700-5p: UAAGGCUCCUUCCUGUGCUUGC, miR-501-3p: AAUGCACCCGGGCAAGGAUUCU. For Western blot, the primary antibodies for beta-actin (SC-47778, Santa Cruz, Dallas, Texas, USA), MUC13 (bs-10074R, Bioss, Boston, Massachusetts, USA), GAPDH (GTX100118, GENETEX, Texas, USA), ZO-1 (ab96587, Abcam, Bristol, UK), and OCC (ab216327, Abcam, Bristol, UK) were used to evaluated protein level.
Transcriptome analysis
Total RNA was extracted using Trizol® Reagent (Invitrogen, USA), and the purified RNA was quantified by using a Bioanalyzer 2100 (Agilent Technology, USA) with RNA 6000 LabChip kit (Agilent Technology, USA). All RNA sample preparation procedures were carried out according to the Illumina’s official protocol. Agilent’s SureSelect Strand-Specific RNA Library Preparation Kit was used for library construction followed by AMPure XP beads (Beckman Coulter, USA) size selection. In flowchart of RNA sequence data analysis, quality trimming was proformed to remove low quality reads/bases by using Trimmomatic51,52. Differential expression analysis was performed using cuffdiff with genome bias detection/correction. Genes were identified that met a > 2-fold change cut-off from among transcriptional regulation with NP treatment. Furthermore, analysis for differential expression genes was carried out53.
Microbiota sequencing
Total genomic DNA from feces samples was extracted using the QIAamp PowerFecal DNA Kit (Qiagen, Hilden, Germany). V3-V4 regions (515F-806R) were amplified, the sequencing library was prepared and sequenced on an Illumina MiSeq PE300 platform. Repeat sequences were organized to generate Circular Consensus Sequencing (CCS)54. CCS data was analyzed using DADA2 to produce Amplicon Sequence Variants (ASVs). Sequencing data were demultiplexed based on barcode and primer sequences using QIIME2 (v2020.11; https://qiime2.org/). Amplicon sequence variants (ASVs) were generated and annotated using the classify-sklearn method trained on the SILVA (v132) or GreenGenes (gg_13_8) databases. Alpha diversity indices, including the Simpson index, were calculated using QIIME2 and analyzed for group differences with parametric (t-test) or non-parametric (Wilcoxon) tests. Diversity metrics were visualized with rarefaction curves generated in R (v3.3.1). Beta diversity was evaluated using principal coordinates analysis (PCoA) and partial least squares discriminant analysis (PLS-DA) plotted in R with the ade4, mixOmics, and ggplot2 packages. Statistical significance of beta diversity differences between groups was determined using parametric (t-test) and non-parametric (Wilcoxon) tests in the agricolae R package. Differential taxa analysis was used Welch’s t-test in STAMP (v2.1.3) and visualized with bar charts. Statistically significant taxa differences (p/q < 0.05) were presented as boxplots. Anosim and MRPP analyses were conducted using the vegan R package.
Sorting and sequencing of intestinal bacteria with NP uptake
Mice were orally administered with NP (100 nm, Fluoresbrite YG carboxylate microspheres) (Polysciences, Warrington, PA, USA). After 2 h, the feces were homogenized (in 1 mL PBS), centrifuged (100 × g for 10 min), filtered (100 μm-mesh sieve), and centrifuged (8000 × g for 10 min) for bacterial pellet collection. Bacteria with NP uptake were sorted using flow cytometry (FACSAria IIIu, Becton Dickinson, Bergen County, NJ, USA), observed using fluorescence microscopy (FV3000, Olympus, Shinjuku city, Japan), and identified by 16S sequencing.
miRNA profile analysis and hybrid prediction
The LS174T cells were incubated in exosome-free medium with contain 1 × 106 particles/mL NP (100 nm) for 48 h, and the LS174T cells-derived EV from culture medium were isolated and collected by ultracentrifugation (100,000 × g; 70 min). Total RNA in mouse intestine and LS174T cells-derived EV were extracted by Trizol® Reagent (Invitrogen, USA) according to the instruction manual. A total amount of 1.2 μg total RNA per sample was used as input material for the small RNA library using TruSeq small RNA Library Prep Kit (Illumina, USA). The libraries were sequenced on an Illumina Novaseq 600 platform, and 75 bp single end reads were generated. Analysis of raw microRNAs-seq data was performed by nf-core/smrnaseq pipeline in Nextflow55. The DEGseq algorithm is used for downstream differential expression analysis56. miRNAs with adjusted p value ≤ 0.05 and ≥ 2-fold changes were considered significantly differentially expressed. Matplotlib, a Python library, was used to draw the heatmap. We further searched these miRNAs targeted genes by using the online resources miRDB. Over-representation analysis was performed to investigate which biological responses and signaling pathways are regulated by those microRNA-targeted genes. MiRDeep2 estimates expression levels of known microRNAs (which reads mapping to miRBase), and also identifies novel microRNAs. Differentially expressed microRNAs were identified that met a > 2-fold change and p-value ≤ 0.05 cut-off among microRNA profiles induced by NP. Furthermore, microRNA targeted genes were identified by using the online resources miRDB. microRNA-targeted genes were investigated that significantly participate in biological responses and regulate signaling pathways via over-representation analysis. The RNAhybrid software was used to calculate the free energy of microRNA-mRNA duplex and predicted miRNA-mRNA hybrid structure57.
Effects of NP, microbial EV, and LS174T-derived EV on microbial growth
The Lachnospiraceae sp. TSD-26 (American Type Culture Collection, ATCC) and Ruminococcaceae sp. TSD-27 (ATCC) were cultured in GS2 medium containing NP (100 nm; 1 × 1010 particles/mL; final concentration).
Lachnospiraceae sp. and Ruminococcaceae sp. were cultured in GS2 medium containing NP for 18 and 44 h, respectively. The culture suspension were collected for EV isolation mediated by centrifugation (10,000 × g for 30 min), filteration (0.22 µm), ultra-high-speed centrifugation (66,226 × g for 1 h), filteration (0.22-µm), ultra-high-speed centrifugation (247,537 × g for 2 h), and pellet resuspended in PBS. Nanoparticle tracking analysis (NTA) (NanoSight LM10-HS, Malvern Instruments, Malvern, UK) was used to determine the number and particle size of the EV41.
The L. paracasei subsp. Paracasei, L. acidophilus, and P. acidilactici were treated with EV (1 × 1010 particles/mL) obtained from Lachnospiraceae sp. or Ruminococcaceae sp. with or without NP induction.
Lachnospiraceae sp. were cultured in GS2 medium containing Ruminococcaceae sp.-derived EV with or without NP treatment, and Ruminococcaceae sp. were cultured in GS2 medium containing Lachnospiraceae sp.-derived EV with or without NP treatment at 37 °C. The L. paracasei subsp. Paracasei, L. acidophilus, P. acidilactici, Lachnospiraceae sp. and Ruminococcaceae sp. were treated with LS174T-derived EV (1 × 1010 particles/mL) with or without NP treatment. The absorbance at 600 nm was measured, and bacterial numbers were counted using bacterial smears.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The raw data generated in this study are provided in the Supplementary Information/Source Data file. Source data are provided with this paper. Source data are available for Figs. 2–4, and 6 and Supplementary Figs. 8 in the associated source data file. Source data are provided with this paper.
References
Chen, X. et al. Chronic exposure to polyvinyl chloride microplastics induces liver injury and gut microbiota dysbiosis based on the integration of liver transcriptome profiles and full-length 16S rRNA sequencing data. Sci. Total Environ. 839, 155984 (2022).
Jing, J. et al. Polystyrene micro-/nanoplastics induced hematopoietic damages via the crosstalk of gut microbiota, metabolites, and cytokines. Environ. Int. 161, 107131 (2022).
Wen, S. et al. Microplastics-perturbed gut microbiota triggered the testicular disorder in male mice: via fecal microbiota transplantation. Environ. Pollut. 309, 119789 (2022).
Jin, Y. X., Lu, L., Tu, W. Q., Luo, T. & Fu, Z. W. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci. Total Environ. 649, 308–317 (2019).
Djouina, M. et al. Oral exposure to polyethylene microplastics alters gut morphology, immune response, and microbiota composition in mice. Environ. Res. 212, 113230 (2022).
Li, B. et al. Polyethylene microplastics affect the distribution of gut microbiota and inflammation development in mice. Chemosphere 244, 125492 (2020).
Sun, H., Chen, N., Yang, X., Xia, Y. & Wu, D. Effects induced by polyethylene microplastics oral exposure on colon mucin release, inflammation, gut microflora composition and metabolism in mice. Ecotoxicol. Environ. Saf. 220, 112340 (2021).
Liu, S., Li, H., Wang, J., Wu, B. & Guo, X. Polystyrene microplastics aggravate inflammatory damage in mice with intestinal immune imbalance. Sci. Total Environ. 833, 155198 (2022).
Jeong, C. B. et al. Microplastic size-dependent toxicity, oxidative stress induction, and p-JNK and p-p38 activation in the monogonont rotifer (Brachionus koreanus). Environ. Sci. Technol. 50, 8849–8857 (2016).
Deatherage, B. L. & Cookson, B. T. Membrane vesicle release in bacteria, eukaryotes, and archaea: a conserved yet under appreciated aspect of microbial life. Infect. Immun. 80, 1948–1957 (2012).
Choi, D. S., Kim, D. K., Kim, Y. K. & Gho, Y. S. Proteomics of extracellular vesicles: exosomes and ectosomes. Mass Spectrom. Rev. 34, 474–490 (2015).
Valadi, H. et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659 (2007).
Mathieu, M., Martin-Jaular, L., Lavieu, G. & Téry, C. Specifcities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 21, 9–17 (2019).
Twu, O. et al. Trichomonas vaginalis exosomes deliver cargo to host cells and mediate host:parasite interactions. PLoS Pathog 9, e1003482 (2013).
Koeppen, K. et al. A novel mechanism of host-pathogen interaction through sRNA in bacterial outer membrane vesicles. PLoS Pathog 12, e1005672 (2016).
Ribeiro, K. S. et al. Proteomic analysis reveals different composition of extracellular vesicles released by two Trypanosoma cruzi strains associated with their distinct interaction with host cells. J. Extracell. Vesicles 7, 1463779 (2018).
Deng, Y., Zhang, Y., Lemos, B. & Ren, H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep. 7, 46687 (2017).
Huang, H., Wei, F., Qiu, S., Xing, B. & Hou, J. Polystyrene microplastics trigger adiposity in mice by remodeling gut microbiota and boosting fatty acid synthesis. Sci. Total Environ. 890, 1642987 (2023).
Jang, Y. J., Kim, W. K., Han, D. H., Lee, K. & Ko, G. Lactobacillus fermentum species ameliorate dextran sulfate sodium-induced colitis by regulating the immune response and altering gut microbiota. Gut Microb. 10, 696–711 (2019).
Chen, X. et al. Polyvinyl chloride microplastics induced gut barrier dysfunction, microbiota dysbiosis and metabolism disorder in adult mice. Ecotoxicol. Environ. Saf. 241, 113809 (2022).
Ahmed, F. E. et al. Diagnostic microRNA markers for screening sporadic human colon cancer and active ulcerative colitis in stool and tissue. Cancer Genomics Proteomics 6, 281–295 (2009).
Link, A., Becker, V., Goel, A., Wex, T. & Malfertheiner, P. Feasibility of fecal microRNAs as novel biomarkers for pancreatic cancer. PLoS One 7, e42933 (2012).
Qiao, J. et al. Perturbation of gut microbiota plays an important role in micro/nanoplastics-induced gut barrier dysfunction. Nanoscale 13, 8806–8816 (2021).
Audie, J. P. et al. Expression of human mucin genes in respiratory, digestive, and reproductive tracts ascertained by in situ hybridization. J. Histochem. Cytochem. 41, 1479–1485 (1993).
Williams, S. J. et al. MUC13, a novel human cell surface mucin expressed by epithelial and hemopoietic cells. J. Biol. Chem. 276, 18327–18336 (2001).
Sheng, Y. H. et al. MUC1 and MUC13 differentially regulate epithelial inflammation in response to inflammatory and infectious stimuli. Mucosal Immunol. 6, 557–568 (2013).
Luo, T. et al. Maternal polystyrene microplastic exposure during gestation and lactation altered metabolic homeostasis in the dams and their f1 and f2 offspring. Environ. Sci. Technol. 53, 10978–10992 (2019).
Sheng, Y. H. et al. The MUC13 cell-surface mucin protects against intestinal inflammation by inhibiting epithelial cell apoptosis. Gut 60, 1661–1670 (2011).
Liu, S. et al. The host shapes the gut microbiota via fecal microRNA. Cell Host Microbe 19, 32–43 (2016).
Díaz-Garrido, N., Badia, J. & Baldomà, L. Microbiota-derived extracellular vesicles in interkingdom communication in the gut. J. Extracell. Vesicles 10, e12161 (2021).
Teng, Y. et al. Plant-derived exosomal microRNAs shape the gut microbiota. Cell Host Microbe 24, 637–652 (2018).
Wang, R. et al. Gut microbiota shape the inflammatory response in mice with an epithelial defect. Gut Microbes 13, 1–18 (2021).
Pothuraju, R. et al. Mucins, gut microbiota, and postbiotics role in colorectal cancer. Gut Microbes 13, 1974795 (2021).
Schooling, S. R. & Beveridge, T. J. Membrane vesicles: an overlooked component of the matrices of biofilms. J. Bacteriol. 188, 5945–5957 (2006).
Ciofu, O., Beveridge, T. J., Kadurugamuwa, J., Walther-Rasmussen, J. & Hoiby, N. Chromosomal beta-lactamase is packaged into membrane vesicles and secreted from Pseudomonas aeruginosa. J. Antimicrob. Chemother. 45, 9–13 (2000).
Mashburn, L. M. & Whiteley, M. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature 437, 422–425 (2005).
Sun, D. et al. Angiogenin maintains gut microbe homeostasis by balancing α-Proteobacteria and Lachnospiraceae. Gut 70, 666–676 (2021).
Kim, K. et al. Role of an unclassified Lachnospiraceae in the pathogenesis of type 2 diabetes: a longitudinal study of the urine microbiome and metabolites. Exp. Mol. Med. 54, 1125–1132 (2022).
Goodrich, J. K. et al. Human genetics shape the gut microbiome. Cell 159, 789–799 (2014).
Luna, R. A. et al. Distinct microbiome-neuroimmune signatures correlate with functional abdominal pain in children with autism spectrum disorder. Cell. Mol. Gastroenterol. Hepatol. 3, 218–230 (2017).
Teng, Y. et al. Gut bacterial isoamylamine promotes age-related cognitive dysfunction by promoting microglial cell death. Cell Host Microbe 30, 944–960 (2022).
Lee, G. et al. Distinct signatures of gut microbiome and metabolites associated with significant fibrosis in non-obese NAFLD. Nat. Commun. 11, 4982 (2020).
Rokhsefat, S., Lin, A. & Comelli, E. M. Mucin-microbiota interaction during postnatal maturation of the intestinal ecosystem: clinical implications. Dig. Dis. Sci. 61, 1473–1486 (2016).
Johansson, M. E., Phillipson, M., Petersson, J., Velcich, A. & Holm, L. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. USA 105, 15064–15069 (2008).
Lu, L., Wan, Z., Luo, T., Fu, Z. & Jin, Y. Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Sci. Total Environ. 631-632, 449–458 (2018).
Wang, M. et al. Oligomer nanoparticle release from polylactic acid plastics catalysed by gut enzymes triggers acute inflammation. Nat. Nanotechnol. 18, 403–411 (2023).
Jiménez‐Arroyo, C., Tamargo, A., Molinero, N. & Moreno‐Arribas, M. V. The gut microbiota, a key to understanding the health implications of micro (nano) plastics and their biodegradation. Microb. Biotechnol. 16, 34–53 (2023).
Hausser, J. & Zavolan, M. Identification and consequences of miRNA-target interactions-beyond repression of gene expression. Nat. Rev. Genet. 15, 599–612 (2014).
Ferruzza, S., Rossi, C., Laura Scarino, M. & Samguy, Y. A protocol for differentiation of human intestinal Caco-2 cells in asymmetric serum-containing medium. Toxicol. In Vitro 26, 1252–1255 (2012).
Singh, R. et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat. Commun 10, 89 (2019).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Kim, D., Langmead, B. & Salzberg, S. L. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360 (2015).
Yu, G., Wang, L. G., Han, Y. & He, Q. Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16, 284–287 (2012).
Fouts, D. E. et al. Next generation sequencing to define prokaryotic and fungal diversity in the bovine rumen. PLoS One 7, e48289 (2012).
Ewels, P. A. et al. The nf-core framework for community-curated bioinformatics pipelines. Nat. Biotechnol. 38, 276–278 (2020).
Wang, L., Feng, Z., Wang, X., Wang, X. & Zhang, X. DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 26, 136–138 (2010).
Remsmeier, M., Steffen, P., Hochsmann, M. & Giegerich, R. Fast and effective prediction of microRNA/target duplexes. RNA 19, 1507–1517 (2004).
Acknowledgements
This research work and subsidiary spending were mainly supported by the Ministry of Science and Technology (MOST) (MOST 110-2628-B-415-001/Excellent Young Scholars Program and MOST 109-2636-B-006-008/Young Scholar Fellowship Program) and Taiwan Food and Drug Administration (MOHW110-FDA-F-114-000374) and supported in part from Higher Education Sprout Project, Ministry of Education to the Headquarters of University Advancement at National Cheng Kung University. We thank the technical services provided by the Bioimaging Core Facility of the National Core Facility for Biopharmaceuticals, Ministry of Science and Technology, Taiwan.
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Walid Mottawea and the other anonymous reviewer for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hsu, WH., Chen, YZ., Chiang, YT. et al. Polystyrene nanoplastics disrupt the intestinal microenvironment by altering bacteria-host interactions through extracellular vesicle-delivered microRNAs. Nat Commun 16, 5026 (2025). https://doi.org/10.1038/s41467-025-59884-y
Received: 13 September 2024
Accepted: 08 May 2025
Published: 10 June 2025
DOI: https://doi.org/10.1038/s41467-025-59884-y
.png)