This week I published an essay in the journal Science on the exciting potential to get ahead of Alzheimer’s disease. That was written a few weeks ago and several important and highly relevant reports have since been published. I’ve incorporated them in this updated version of how we can move forward to prevent this dreaded disease.
With all the advances in both the science of aging and artificial intelligence (AI), we are in a propitious position to accurately and precisely determine who is at high risk of developing Alzheimer’s disease years before signs of even mild cognitive deficit. It takes at least 20 years for aggregates of misfolded β-amyloid and tau proteins to accumulate in the brain along with neuroinflammation that they incite. This provides a long window of opportunity to get ahead of the pathobiological process, both for prediction and prevention.
A family history of Alzheimer’s and the presence of genetic variants in the APOE4 (apolipoprotein E4) allele indicate an increased risk, as does a polygenic risk score that is based on the combined influence of many genetic variants. However, each of these clues provides little insight about when initial symptoms would likely present. After all, there is a world of difference between whether a person is at risk of manifesting the disease at age 98 instead of 68. Now there are a number of better tools that have sharpened our predictive capacity. At the body-wide level, measuring the degree of DNA methylation can serve as a kind of clock, indicating whether biological age is outpacing chronological age—a predictor of dementia. Fast-paced aging measures by eight so-called organ clocks, including ones for the brain and immune system, have been shown to predict adverse health outcomes, including Alzheimer’s disease.
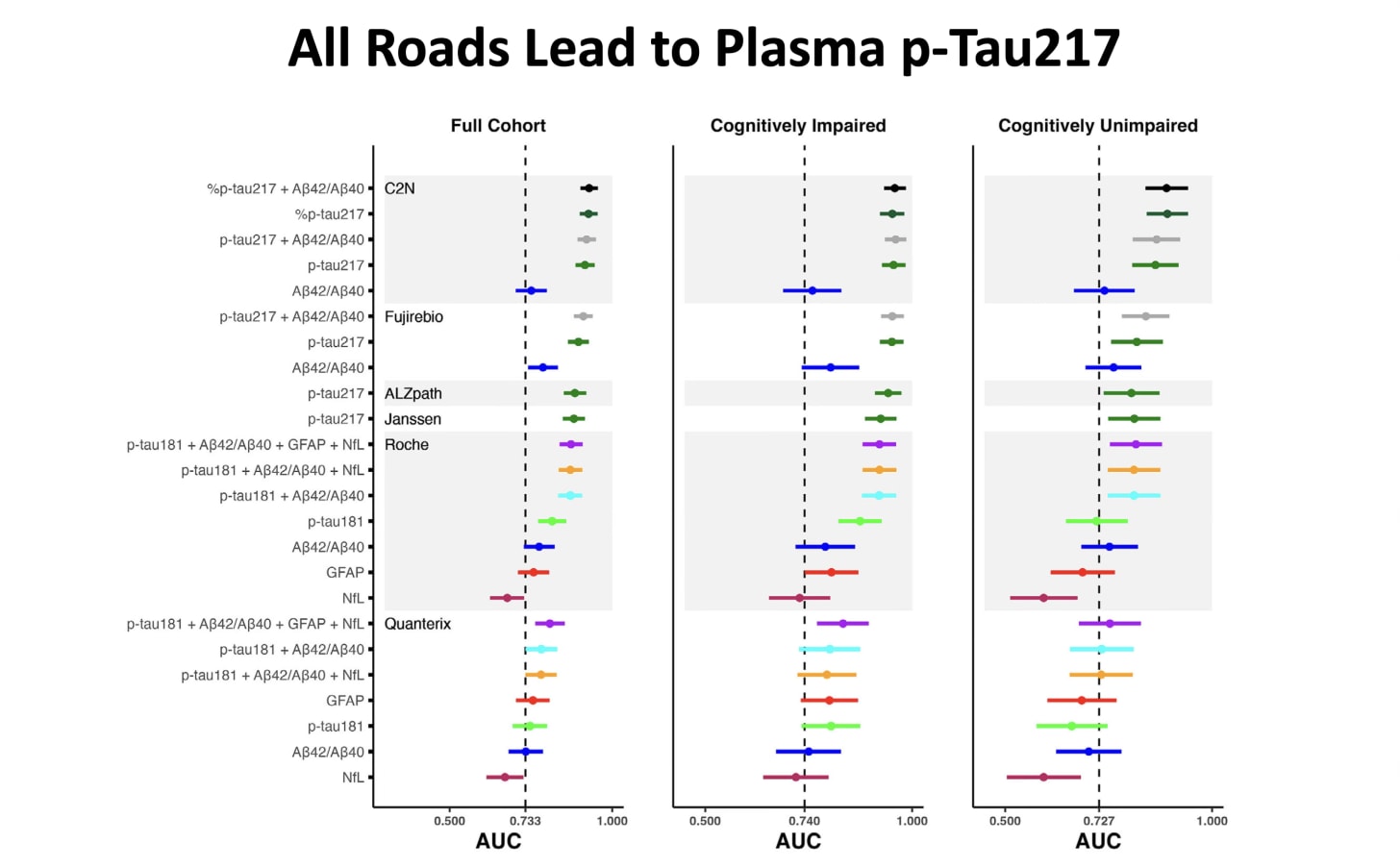
Beyond the hundreds of plasma proteins that can serve as a brain clock, there are specific blood-based protein biomarkers. An assay that measures the amount of a misfolded protein called p-tau217 (tau phosphorylated at threonine-217) accurately differentiates Alzheimer’s from other forms of dementia. [I wrote about this breakthrough test in a previous Ground Truths.] Although several other blood biomarkers have been reported, p-tau217 is the earliest to increase in the long course of disease development and has consistently excelled in the accuracy and specificity of its predictions. It has been shown to be as good as or superior to p-tau217 assessed from cerebrospinal fluid, which requires a lumbar puncture, and as accurate as a positron emission tomography (PET) tau scan. The Figure below shows it to be the best of all comparative plasma biomarkers. Furthermore, this biomarker provides a temporal window for flagging the risk of the disease multiple years in advance. Other protein markers have also been correlated with risk (including p-tau181, microtubule-binding region containing residue 243, glial fibrillary acid protein, neurofilament light chain, and plasma proteins such as growth differentiation factor 15). A group of these could be used in a panel to back up the finding of p-tau217. While p-tau217 has been available at LabCorp and Quest, among other labs, for the past 2 years, one test from the Japanese company Fujirebio (measuring levels of p-tau 217 and beta-amyloid 1-42) was cleared by the FDA to facilitate diagnosis in patients with symptoms. By report, 92% of individuals with positive blood-test results had the presence of amyloid plaques confirmed by either a PET scan or a spinal-fluid test, while 97% of individuals with negative results from the blood test also had confirmed negative results using the other methods
A new study of a cohort of 400 middle-aged participants with intact cognitive function and extended follow-up for Alzheimer’s disease again showed the accuracy of p-tau217 and its ratio p-tau217/Aβ42 for discriminating PET Aβ scan status.
A new paper in Nature Aging assessed nearly 7,000 unique plasma proteins (Somascan, see prior Ground Truths on high-throughput plasma proteins and A.I.) in ~1,300 participants with Alzheimer’s and ~2,100 who were cognitively intact. The analysis using machine learning identified several proteins that predicted clinical AD and biomarker AD status.
Again the value of a plasma p-tau217 test compared with cerebrospinal fluid or amyloid PET scan was confirmed.
On another front, AI offers the possibility of predicting Alzheimer’s with retinal images from a photograph or by optical computed tomography. When analyzed by deep learning AI, the images can forecast the risk of Alzheimer’s in the next 5 years among people who are asymptomatic. Moreover, layers of data from brain clocks, biomarkers, and other tests can be integrated in a multimodal AI model to determine whether a person is at high risk of Alzheimer’s and, if so, project a timeline for the disease’s progression.
Once an individual has been accurately identified as having a high risk for Alzheimer’s, there are now many means of prevention, or at the least, substantial deferral. Lifestyle factors, such as exercise, have been demonstrated to substantially lower p-tau217, which may signify a preventive strategy akin to lowering low-density lipoprotein (LDL) cholesterol for prevention of heart disease. In a recent study following more than 100,000 participants for 30 years, only 9% reached age 70 without chronic diseases, including Alzheimer’s. Their diet was rich in fruits, vegetables, and grains and low in red meat, and otherwise Mediterranean-like. A recent randomized trial of participants with elevated p-tau217 and other biomarkers indicated that lifestyle improvements including a healthy diet, loss of weight, and exercise lowered risk. Multiple studies have emphasized the importance of healthy sleep habits, including the amount of deep sleep, when clearance of the brain’s metabolic waste products occurs.
The recent Lancet Commission on dementia concluded that about 70% of cases are caused by Alzheimer’s disease, and that 45% of them are preventable. The strategies for prevention, beyond lifestyle factors involved with p-tau217 reduction, include management of hypertension, diabetes, high cholesterol, and obesity, as well as treatment for hearing and vision loss, avoidance of excessive alcohol, reduction of intake of ultraprocessed foods, and cessation of smoking.
Recently, three impressive natural experiments—observational studies of naturally occurring, real-world events—all pointed to the role of shingles vaccination in protection from dementia [Reviewed here]. Other observational studies have also indicated that influenza vaccines can protect against Alzheimer’s disease. Although these findings do not provide any proof of a viral basis for Alzheimer’s, they do support the notion that revving up the immune system may help stave off the disease.
When a person is identified at high risk for Alzheimer’s, aggressive surveillance can be undertaken using the same baseline assessments that established risk. That includes further tests involving the brain organ clock, AI analysis of the retina, p-tau217 and other biomarkers, and, if necessary, brain imaging with either magnetic resonance imaging (MRI) or PET.
Multiple observational studies have indicated a reduction of risk for Azheimer’s with the use of GLP-1 (glucagon peptide-1) drugs, which are now being tested in randomized trials. If they pan out, this may be because of their strong anti-inflammatory effects in the brain. A new experimental model study demonstrated a GLP-1 drug favorable effect of less cognitive decline by restoring cellular energy metabolism and improving microglial phagocytosis (schematic below)
Besides the interventions reviewed above, considerable work is ongoing to manipulate the gut microbiome. Studies in which gut microorganisms are transplanted from both people with Alzheimer’s disease and control subjects to young heathy rats indicate that the composition of the gut microbiome is a risk marker for the disease. Also in the pipeline for prevention of Alzheimer’s disease in people at high risk are potent oral medications with high penetrance to the brain, such as one that targets NLRP3, a protein involved in inflammation.
With all of these innovations, there has never been a greater opportunity to identify individuals at high risk for Alzheimer’s and prevent them from developing the disease, an overall strategy that would be far preferable to trying to cure or treat it once cognitive impairment has already begun.
_____________________________________
BTW, I have a chapter in my book SUPER AGERS that gets into much more depth for how we can use the p-tau 217 blood biomarker along with all of a person’s layers of data to go into aggressive prevent mode. I want to thank many of you subscribers of this newsletter who have helped put it on the NYT bestseller list and the top non-fiction new release book on Amazon.
******************************************
2 Quick Polls
Thanks for reading and subscribing to Ground Truths.
If you found this interesting please share it!
That makes the work involved in putting these together especially worthwhile.
All content on Ground Truths—its newsletters, analyses, and podcasts, are free, open-access.
Paid subscriptions are voluntary and all proceeds from them go to support Scripps Research. They do allow for posting comments and questions, which I do my best to respond to. Many thanks to those who have contributed—they have greatly helped fund our summer internship programs for the past two years.
.png)







