Introduction
Urine recycling is gaining traction as an approach for sustainable wastewater management1,2,3,4,5. Although urine composes only 1% of total wastewater, it contains roughly 70–90% of nitrogen (N) and 50–65% of phosphorus (P) in waste streams6,7, leading to environmental issues such as eutrophication. Because urine is relatively easy to separate from other waste streams, researchers are exploring decentralized urine diversion (UD) processes for their economic and environmental benefits over traditional centralized processes4,5. In UD processes, urine is concentrated to produce N, P, and potassium (K)-based fertilizers for local agriculture. If fully utilized, urine could replace 21%, 12%, and 20% of global N-, P-, and K-fertilizer demands, respectively7,8,9. Additionally, city-wide UD processes could reduce greenhouse gas emissions, energy demands, freshwater usage, and eutrophication potential by 20–60% compared to conventional centralized processes10. These processes may prove crucial for the sustainable growth of our economy.
Two main scenarios for UD processes are often considered: urine concentration (UC) and struvite and ammonium sulfate (SAS) processes10. The UC process uses reverse osmosis (RO) to concentrate urine and produce urea- and struvite (NH4MgPO4·6H2O)-based fertilizers. Due to the rapid decomposition of urea to ammonia by environmental microbes, the SAS process involves adding Mg salts for struvite production and using ion exchange to recover ammonium salts. While producing chemical fertilizers from urine is environmentally beneficial, their relatively low market prices (USD 300-400/ton) limit economic incentives. The success of UD processes hinges on fully exploiting the potential of urine to produce as many value-added products as possible. To enhance the economic attractiveness of UD processes, we sought to produce other high-value commodity chemicals from urine.
Among the chemicals in urine, urea stands out due to its abundance and its potential to induce biomineralization, making hydroxyapatite (HAp) an ideal target11. HAp, a calcium phosphate mineral with the chemical formula Ca5(PO4)3OH, is a major component of biocomposites such as bone and teeth in vertebrates12 and impact-resistant shells in some marine invertebrates13,14. Due to its biocompatibility, HAp is extensively used in orthopedic, oral care, and plastic surgery applications, as well as in the restoration and protection of archaeological materials. HAp’s high capacity to absorb fluoride and heavy metals makes it valuable for water purification and industrial downstream processing15,16. The market for HAp is projected to exceed USD 3.5 billion by 2030, with a high sales price (over USD 80 per kg) enhancing the economic attractiveness of UD processes. HAp composites, known for their lightweight, high mechanical strength, toughness, and durability, have the potential to serve as renewable and biodegradable alternatives to various commodity materials like plastics and building materials17,18,19,20,21,22,23. Reducing the cost of HAp production could also lower the carbon and energy footprints of these processes, facilitating the global expansion of UD processes.
In this study, we develop a synthetic yeast platform osteoyeast for HAp production directly from urine, inspired by the biological mechanisms of osteoblasts (Fig. 1a)24. Using correlative optical and electron microscopy, we show that osteoyeast mimics osteoblast-like mineralization behavior by accumulating and secreting amorphous calcium phosphate (ACP), which crystallizes into HAp. The engineered yeast efficiently synthesizes HAp from urine, achieving titer exceeding 1 g/L. A techno-economic analysis demonstrates that this bioprocess offers significant cost and sustainability advantages over conventional urine diversion strategies. These findings establish osteoyeast as a promising platform for high-value biomanufacturing and resource recovery from human waste.
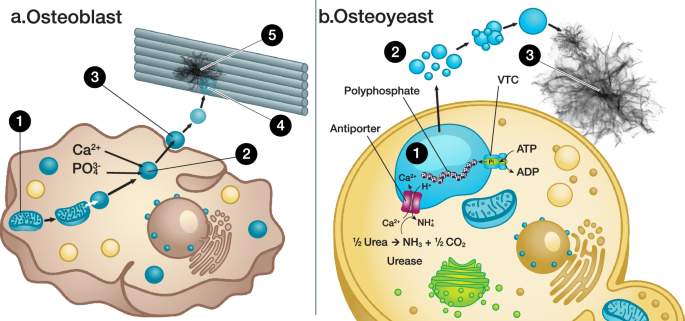
a Hydroxyapatite (HAp) synthesis catalyzed by osteoblasts. Dense calcium phosphate particles are formed in mitochondria, and they interact with lysosomes (Step 1). Osteoblasts start to transport, accumulate, and store phosphate and calcium within their lysosomes (Step 2). As the lysosomes are filled with amorphous calcium phosphate (ACP), they are secreted into the extracellular milieu as matrix vesicles (MVs) (Step 3). These MVs interact with proteins such as collagen and are gradually deformed, releasing ACP (Step 4). Lastly, platelet-like HAp is formed within the gaps of collagen fibrils, which act as templates (Step 5). b The design principle used for engineering the osteoyeast platform. The ureolytic enzyme is overexpressed, which triggers the activity of an antiporter (Vcx1) to exchange cytosolic Ca2+ with intra-vacuolar H+. Ca2+ is accumulated and stored in the form of ACP (Step 1). The ACP in the vacuoles is translocated to extracellular vesicles (EVs) and secreted into the media (Step 2). The EVs merge, and ACP inside the EVs transforms into HAp (Step 3). VTC vacuolar transporter chaperone.
Results
Design principle
We leveraged the underlying biological mechanisms of HAp synthesis by osteoblasts25,26,27,28,29,30 as the foundation for developing a design principle to engineer our osteoyeast strains (Fig. 1). In osteoblasts, HAp synthesis occurs through a series of several distinct steps. First, dense calcium phosphate particles are formed in the mitochondria and subsequently interact with lysosomes. Next, osteoblasts transport, accumulate, and store calcium and phosphate in their lysosomes, acidic organelles critical for maintaining organismal homeostasis28. These lysosomes contain polyphosphate29, which, along with their low pH, likely stabilizes the ACP phase30. Once filled with ACP, lysosomes are secreted into the extracellular environment as matrix vesicles (MVs), where polyphosphate is gradually replaced with monophosphate. These MVs interact with structural proteins such as collagen, deform over time to release ACP, and ultimately facilitate the formation of platelet-like HAp crystals within collagen fibril gaps, using the fibrils as templates for mineralization.
To engineer osteoyeast, Saccharomyces boulardii was selected as the chassis due to its greater tolerance to pH variation compared to S. cerevisiae31. The yeast vacuole, a lysosome-like acidic organelle responsible for pH homeostasis, ion storage, and metal tolerance, was chosen as the primary engineering target (Fig. 1b)32. The vacuole contains polyphosphate synthesized by the vacuolar transporter chaperone (VTC) complex33, while the H+ antiporter Vcx1 facilitates calcium transport and intracellular pH regulation34,35,36. We hypothesized that increasing cytosolic pH through the production of hydroxide ions, generated via urea decomposition by ureolytic enzymes such as urea amidolyase, would activate Vcx1 to pump H+ out of the vacuole to help neutralize cytosolic pH. Consequently, calcium ions are accumulated in the vacuoles as ACP (Fig. 1b, Step 1). Although the precise mechanisms remain unclear, various fungal species are known to produce extracellular vesicles (EVs) through diverse pathways. These include vesicle-containing vacuoles37, Golgi apparatus-mediated secretory pathways38, and the endosomal sorting complex required for transport (ESCRT) machinery39,40. These pathways present an opportunity to engineer the secretion of ACP via EVs (Fig. 1b, Step 2). Once secreted, polyphosphate in EVs degrades to monophosphate catalyzed by Ppn1/2 and is not replenished by VTC due to the absence of ATP41,42. The abiotic conversion of ACP to HAp occurs under suitable environmental conditions (Fig. 1b, Step 3). This systematic approach underpins our strategy to develop a yeast-based platform for HAp synthesis.
Accumulation of calcium in vacuoles
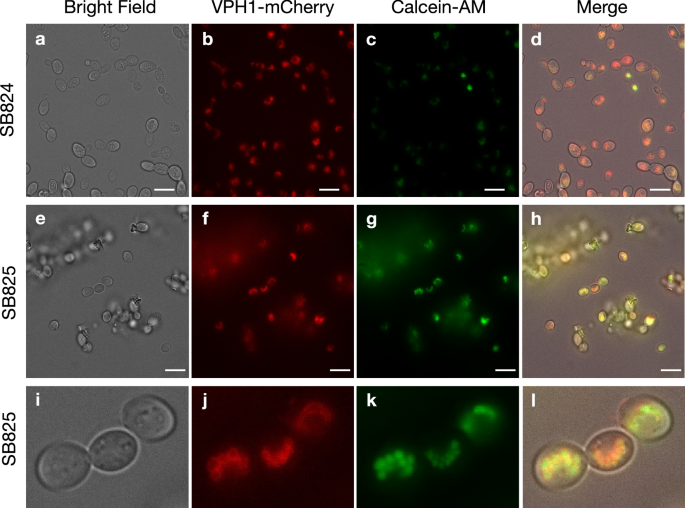
We first engineered the S. boulardii strain (SB760) to constitutively express genes for a urea amidolyase (dur12) (strain SB818), and both dur12 and a urea transporter (dur3) (strains SB822-SB823) (Table 1). Testing the urease activity of SB760, 818, and 822 (Supplementary Fig. 1) revealed that only SB822 showed increased urease activity, indicating that both dur12 and dur3 are required for urea decomposition. To confirm SB822’s ability to accumulate calcium in vacuoles, we used brightfield and wide-field fluorescence microscopy during urea decomposition. We visualized the vacuole membrane by expressing a gene coding for V-type proton ATPase subunit A (Vph1) fused with an mCherry fluorescent protein, creating strains SB824-SB825 (Table 1)43. We also used calcein-acetoxymethylester (calcein-AM) to monitor intracellular calcium accumulation, as membrane-permeable calcein-AM is hydrolyzed by intracellular esterases to impermeable calcein, which fluoresces upon binding to calcium ions44. SB824 and SB825 were inoculated in modified SD media (no ammonium sulfate, 50 mM Ca2+, and 20 g/L urea) and grown for 18.5 h at 37 °C. SB825 increased the culture pH to 6.06, while SB824 did not (pH 3.97). After adding calcein-AM to each culture, fluorescence microscopy showed that calcein accumulated in the vacuoles of both strains, with stronger signals in SB825, indicating higher calcium accumulation (Fig. 2). These results indicated successful engineering of vacuoles to transport, accumulate, and store Ca2+. Interestingly, crystal-like substances appeared only in SB825 cultures, suggesting its involvement in their formation.
The optical images of yeast strains SB824 (a–d) and SB825 (e–h) grown in the YNB media with 20 g/L urea, 50 mM Ca2+, and 10 µM calcein-AM are shown. Three yeast cells located at the center of the panels (box in h) are magnified and shown (i–l). Bright field (a, e, i), channel for VPH1-mCherry (b, f, j), channel for Ca2+-bound calcein (c, g, k), and merged (d, h, i) images are shown. The calcein channel contrast was set to the same minimum and maximum for each strain for comparison. The scale bar represents 5 μm in (a–h). A portion of panels (e–h) was magnified 4.5× and presented in panels (i–l). The experiment was performed with at least ten biological replicates, all yielding similar results. Representative data are shown.
HAp synthesis
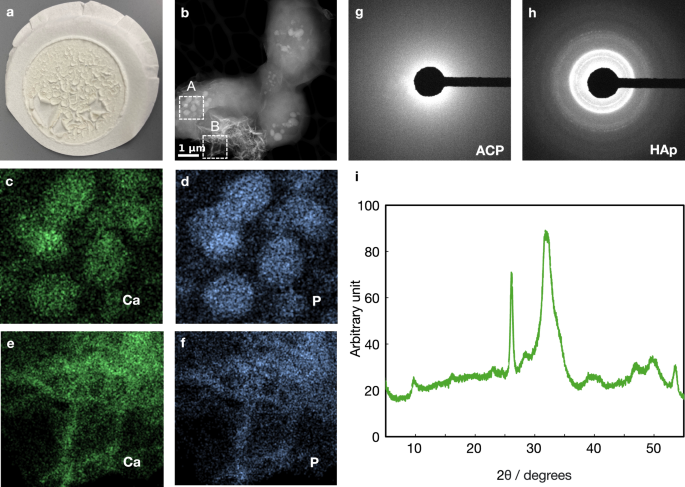
To further characterize the vacuolar content and extracellular crystal-like substances, we analyzed the SB822 culture using transmission electron microscopy (TEM). After cultivating SB822 in modified YNB media for less than 24 h, white precipitants were visible and removed (Fig. 3a). This material was washed with deionized water, dried, and analyzed using high-angle annular dark-field scanning TEM (HAADF-STEM) imaging (Fig. 3b). This analysis revealed that the precipitants were mixtures of cells and inorganic substances in different phases. The intracellular particles and platelet-like extracellular crystals likely represented Ca2+ accumulated in the vacuoles and crystal-like substances, respectively (Fig. 3b, zones A and B). Elemental analysis using STEM-energy-dispersive X-ray (EDX) spectroscopy of zones A and B (indicated in Fig. 3b) showed that both substances were composed mainly of calcium and phosphorus (Fig. 3c–f and Supplementary Fig. 2). Selected-area electron diffraction analysis further confirmed that the intracellular particles were filled with ACP (Fig. 3g) and the extracellular platelet-like crystals were crystalline HAp (Fig. 3h). Notably, polyphosphate and low pH in vacuoles might also play an important role in maintaining the amorphous form of calcium phosphate. The crystal components were further separated by gradient centrifugation. X-ray diffraction (XRD) analysis showed that the HAp produced by the engineered strain was very similar to that of bone (Fig. 3i)45. Together with the results from fluorescence microscopy, these findings indicated that we had successfully engineered yeast vacuoles to transport, accumulate, and store calcium in the form of ACP, creating a yeast strain capable of mediating bone-like HAp synthesis, which we named osteoyeast.
a Material produced by the osteoyeast strain SB822, collected using 10 μm filter paper. b HAADF-STEM image of the collected material. STEM-EDX elemental maps for Ca (c) and P (d) in zone A, and Ca (e) and P (f) in zone B. g, h Electron diffraction patterns corresponding to zone A and B, respectively. i XRD analysis of HAp synthesized by osteoyeast, showing a pattern comparable to that of bone. The experiment was performed with at least ten biological replicates, all yielding similar results. Representative data are shown.
Secretion of ACP EVs
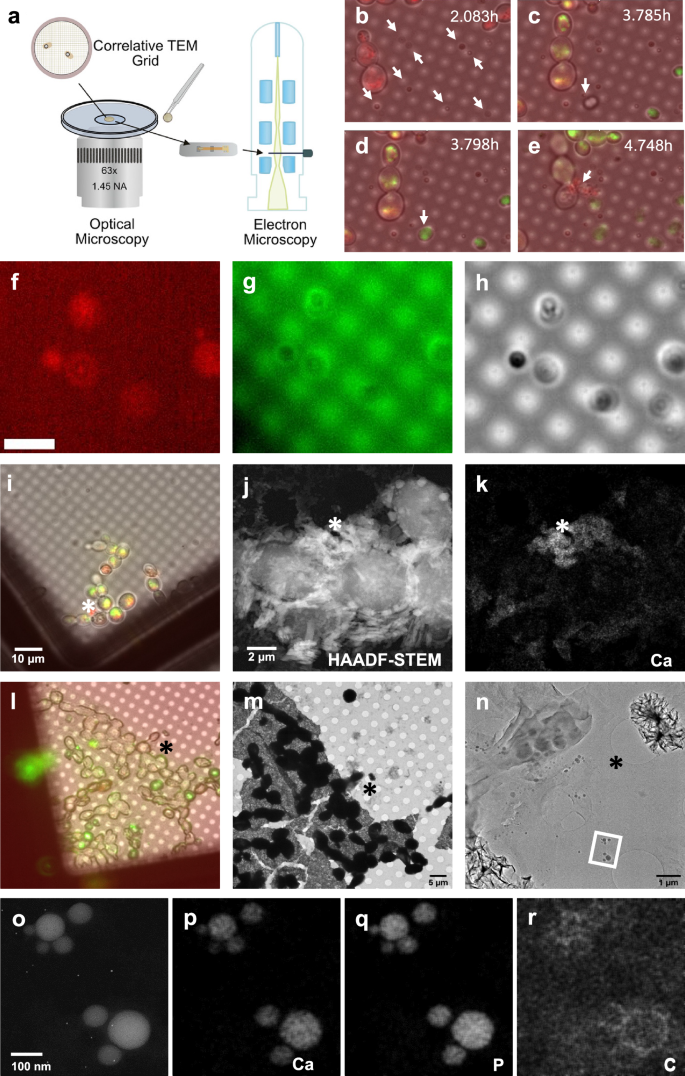
To directly correlate the phenomena observed using fluorescence microscopy and TEM, we developed a correlative imaging method to monitor the cellular processes underlying HAp synthesis (Fig. 4a). This method uses reference/finder TEM grids to locate the same cells. First, we used fluorescence microscopy to monitor the cellular processes for intracellular ACP formation and extracellular HAp synthesis. Next, we dried the samples on the grids and analyzed them using TEM. Although the samples moved slightly during drying, the relative locations of each cell and HAp crystal corresponded well enough to allow for the correlation of the two different imaging modes. In the first step, the SB825 strain was grown in modified YNB media on the TEM grid. We took pictures every 45 s using fluorescence microscopy and created a movie describing the cellular processes (Supplementary Movie 1). Figure 4b–e show snapshots of the important events during HAp synthesis. As expected, the SB825 strain first accumulated calcein within the vacuoles. During this time, we noticed that small particles appeared around the yeast cells at 2.083 h (Fig. 4b, arrows). Between 3.760 and 3.823 h (Supplementary Movie 1 and Fig. 4c, d), many HAp-like particles were formed. Notably, we observed the fusion of two EVs at 3.785 h (Fig. 4c), and this larger EV was transformed into HAp-like particles at 3.798 h (Fig. 4d). Although the intensity was low, we also found that these particles were fluorescent in the mCherry channel, suggesting that they were derived from the vacuoles (Fig. 4f–h). Interestingly, the nascent HAp-like particles showed high calcein signals (Fig. 4c, d), but these signals gradually decreased over time (Fig. 4e), possibly indicating the transformation of ACP into HAp.
a Scheme for correlative imaging analysis. b–e The time course of HAp synthesis mediated by the osteoyeast platform. The arrows indicate (b) emergence of extracellular vesicles, (c) fusion of two extracellular vesicles to a single vesicle, (d) conversion to HAp, and (e) exudate from the ruptured yeast cell. f–h Optical imaging of extracellular vesicles and HAP-like particles. The EVs and HAP-like particles appear in the mCherry channel (f) and the calcein channel (g) and are observable by bright-field imaging (h). i–n Analysis of a ruptured yeast cell during HAp synthesis using fluorescence microscopy. j A HAADF-STEM analysis of a ruptured yeast cell. k A STEM-EDX analysis of Ca from the ruptured yeast cell. i–k The asterisks denote the same locations analyzed using different microscopy methods. l Analysis of yeast cells during HAp synthesis using fluorescence microscopy. m, n TEM analysis of (l) with increased magnification. l–n The asterisks denote the same locations analyzed using different microscopy methods. o A HAADF-STEM analysis of the zone denoted with the rectangle. p–r STEM-EDX analyses of Ca, P, and C. The experiment was performed with at least three biological replicates, all yielding similar results. Representative data are shown.
At 4.748 h, one of the yeast cells underwent necrosis, and the cell contents were exuded (Fig. 4e). The exudate was also covered with red fluorescence, suggesting that vacuoles were translocated outside the cells. We therefore investigated this exudate as a possible mechanism for HAp synthesis (Fig. 4i–k). However, correlative TEM analysis suggested that the exudate contained ACP rather than HAp (Fig. 4l–n). Additionally, the proportion of yeast cells that underwent necrosis was low, ~1 cell per 200 cells, indicating that necrosis is unlikely the primary mechanism for ACP translocation. Further TEM analysis showed many EVs ~20 to 150 nm in diameter, around the yeast cells (Fig. 4n). STEM-EDX and electron diffraction revealed that these EVs were filled with ACP (Fig. 4o–r). The presence of carbon around each ACP particle suggests that the particles were encapsulated in lipid membranes (Fig. 4r). The TEM image also showed that several EVs gathered closely and may have been about to fuse into larger EVs. Our results suggest that yeast cells can translocate vacuolar ACP into EVs, which are then secreted. Although the mCherry signal was of low intensity, EVs also carried mCherry signals on their membranes (Fig. 4f–h), suggesting an association with vacuoles. However, the dilution of red fluorescence may mean that a fraction of the vacuoles might fuse with another organelle or the plasma membrane during EV formation. After being secreted, EVs could merge into larger EVs, and ACP in the EVs could be transformed into HAp if a certain size is reached or if the media pH becomes high enough to catalyze the transformation of ACP into HAp.
Transformation of ACP EVs into HAp
In cells, polyphosphate within vacuoles undergoes a dynamic cycle of replenishment. The Ppn1/2 enzymes decompose polyphosphate, while the VTC complex synthesizes polyphosphate using ATP as a substrate. Once extracellular vesicles (EVs) are secreted into the medium, ATP is no longer available, leading to the degradation of polyphosphate into monophosphate. This degradation plays a critical role in crystal synthesis, as polyphosphate likely inhibits crystal formation. Notably, significant calcein fluorescence signals were detected following HAp formation between 3.785 and 3.798 h (Fig. 4c, d), indicating the presence of loosely bound calcium within the HAp crystals. Over time, the calcein signal diminished (Fig. 4e), suggesting that free calcium was progressively incorporated into the HAp crystal structure or released into the surrounding medium.
Furthermore, the transformation of ACP within EVs into HAp particles occurred simultaneously (Fig. 4c, d). This transformation appears to be triggered by pH changes during fermentation, which occur as the culture pH rises over time due to urea degradation. This process mirrors the co-precipitation method commonly used for HAp production. To investigate this, a time-course experiment was conducted to monitor the relationship between pH changes and HAp formation during fermentation (Supplementary Fig. 3). Initially, the culture pH was 4.4. After 15 h of fermentation, the pH increased to 5.8, and no visible crystals were observed. Approximately 30 min later, crystal flakes began to form in the culture, coinciding with a drop in pH to 5.2. In this pH range, phosphate predominantly exists as H2PO4−. The observed pH drop is likely associated with HAp crystallization, driven by the deprotonation of H2PO4−. By 92 h, the pH had further increased to 8.2.
TEM analysis of samples collected at different pH levels revealed distinct structural changes. At pH 5.8, ACP vesicles, which likely represent vacuoles, were observed (Supplementary Fig. 3a). At pH 5.2, platelet-like HAp crystals began to form (Supplementary Fig. 3b), and by pH 8.2, flower-like HAp structures were present in the culture (Supplementary Fig. 3c). Interestingly, HAp formation occurred at a relatively low pH, despite conventional co-precipitation methods typically requiring higher pH values (10–11). In vertebrates, HAp formation is templated by collagen at physiological pH. Similarly, it is plausible that macromolecules secreted alongside ACP vesicles serve as templates for HAp nucleation and crystallization, enabling the process to occur under these conditions. To illustrate these findings, we have summarized the comparison of wild-type and engineered S. boulardii strains for HAp synthesis as schematic diagrams (Supplementary Fig. 4).
HAp synthesis from urine
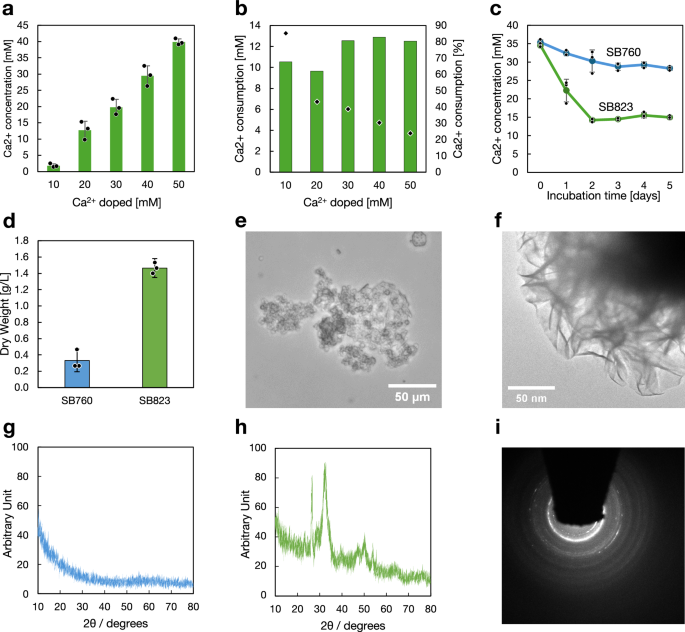
We tested the osteoyeast’s ability to produce HAp directly from human urine (purchased from Innovative Research Inc.). The pH of the urine was adjusted to 5.5 using acetate, a level optimal for yeast cultivation but inhibitory to bacterial growth, thereby slowing urea decomposition. Various concentrations of CaCl2 (10–50 mM) were added to the urine, as the calcium concentration in urine varied between 2.5 and 5.0 mM from batch to batch. Fermentation was initiated by inoculating the SB823 strain at a final OD of 0.1. The cultures were grown for 5 days, after which the remaining calcium concentrations were measured (Fig. 5a). Based on these measurements, total calcium consumption and the percentage of calcium consumed were calculated (Fig. 5b). When osteoyeast was cultivated in urine supplemented with an additional 10 mM calcium, it consumed over 10 mM of calcium, corresponding to 85% of the total calcium. However, when cultured in urine supplemented with 40 mM calcium, osteoyeast consumed only 13 mM, and the consumption rate dropped to about 30%.
a Remaining calcium concentration in the cultures 5 days after the fermentation began. b Calculated calcium consumption amount (bars) and percentage (plot) based on the samples in (a). c Calcium consumption in the cultures. The experiment shown in (a) was repeated using the higher inoculum size. d Dry weight of pellets collected from cultures grown for 10 days. e An optical image of the SB823 culture. f HAADF-STEM analysis of the SB823 culture. g, h XRD spectra of the dried pellets from the SB760 and SB823 cultures. i Electron diffraction analysis of the product identified in (f). Each dot (a, c, d) represents an individual measurement from a biological triplicate. Bars (a, b, d) and lines (c) indicate the mean values, and error bars represent the standard deviation (a, c, d). Source data are provided as a Source Data file.
We observed that calcium consumption plateaued at 12, 13 mM, even when higher calcium concentrations were added. We hypothesized that this limitation could be due to the inoculum size. To test this, we increased the inoculum to a final OD of 0.15 and selected urine supplemented with 30 mM calcium for a time-course experiment using SB823 and its control strain, SB760 (Fig. 5c). Calcium consumption occurred at a rate of 12 mM/day, with osteoyeast ceasing calcium consumption two days after fermentation began. On day 10, the cultures were terminated, and the precipitates were collected by centrifugation. The precipitates were oven-dried, and HAp production was estimated to be ~1.1 g/L compared to the control sample (Fig. 5d). Although precise biomass measurements in SB823 cultures were not possible, a growth comparison between SB760 and SB823 in mYNB medium without calcium suggests that the biomass yield of SB823 was reduced to ~30% of that of SB760 (Supplementary Fig. 5). This observation supports the validity of our analysis. The sample was further analyzed using optical microscopy, XRD, TEM, and electron diffraction, confirming HAp synthesis (Fig. 5e–i).
TEA of HAp synthesis
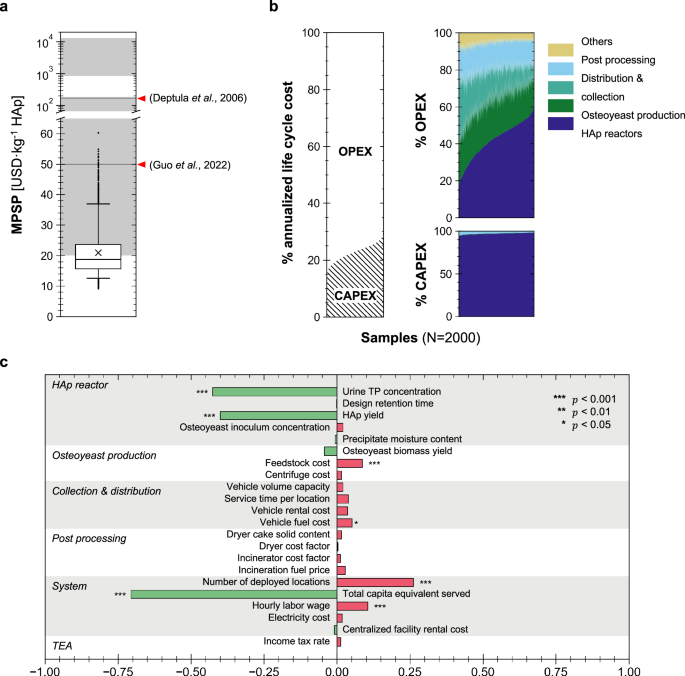
To investigate the financial viability of HAp synthesis from urine, we designed, simulated, and conducted a techno-economic analysis (TEA) of a city-scale HAp production system using the open-source process simulators QSDsan46,47 and BioSTEAM48,49,50. Given the transient nature of urea in fresh, undiluted human urine and the volatility of ammonia51, we assumed that HAp synthesis reactors would be distributed across a densely populated city such as San Francisco and serve somewhere between 10,000 and 80,000 people. Precipitates from the reactors would be regularly collected, transported, and processed into HAp products at a centralized facility. Osteoyeast would be cultivated centrally and distributed to the reactors. The TEA system boundary includes the HAp synthesis reactors, city-wide precipitate collection and material distribution system, centralized osteoyeast production, HAp processing, and auxiliary facilities. A summary of end-to-end mass balance, unit operation design, and equipment costing of the proposed system is included in the Supplementary Materials. The minimum product selling price (MPSP) of HAp for the system to break even was chosen as the indicator of financial viability. Monte Carlo simulations (n = 2000) were performed to incorporate the uncertainty of process design, system performance, and deployment contexts into MPSP evaluation, and Spearman’s rank correlation coefficients were characterized to determine the sensitivity of MPSP to each individual assumption.
Despite the uncertainty, the TEA demonstrated this system offers a potentially profitable approach for sustainable HAp production. At the simulated scales, the system can recover P from urine and produce 65.4 tonne HAp per year (median value, the 5th–95th percentiles were 18.7–127 tonne/year, which will be presented in brackets hereinafter). The median MPSP from Monte Carlo simulations was 18.8 (12.6–36.9) USD/kg HAp (Fig. 6a), all within or below the market price ranges of HAp at various grades (Supplementary Data 1–6)52,53,54. Operating expenses (OPEX) over the 10-year project lifetime account for 68.3–87.6% of the system’s life cycle cost (Fig. 6b). The system’s deployment context drives the onsite operating cost of these reactors and the costs of logistics, contributing 59.2% to the total OPEX. Osteoyeast production and precipitate post-processing also significantly contribute to OPEX. Sensitivity analysis demonstrated that contextual factors and technical performance of the HAp synthesis process are the most impactful drivers for the MPSP (Fig. 6c). Covering a larger population or increasing centralization can lower the unit cost of HAp production.
a Minimum product selling price (MPSP) of the recovered HAp. Whiskers, boxes, and the middle line represent 5th/95th, 25th/75th, and 50th percentiles, respectively, from Monte Carlo simulation samples (n = 2000). Each sample represents a unique stochastic realization of the TEA model (i.e., technical replicates under varying input parameters). “×” indicates the mean MPSP of all samples. The shaded gray regions indicate the market price ranges for HAp of various grades (e.g., food, industrial, cosmetic, medical; 90-99.5% purity; detailed in Supplementary Data 4). Horizontal gray lines indicate price values from literature73,74. b Breakdown of the system’s life cycle cost into operating expenses (OPEX) and capital expenditures (CAPEX). Samples were sorted with ascending contributions of a single category for better visualization. c The relative sensitivity of HAp MPSP to different parameters, indicated by the magnitudes of the Spearman’s rank correlation coefficients (\(\rho\)) between MPSP and individual parameters. The null hypotheses of no monotonic relationship between MPSP and individual parameters (i.e., \(\rho=0\)) were tested using two-sided t-approximations (df = 1998). Exact \(\rho\) and p values are provided in Supplementary Data 5 and 6. Source data are provided as a Source Data file.
The median unit cost of urine treatment with the HAp synthesis system was estimated to be 54.5 USD/m³ urine. Assuming the recovered HAp was sold at 50USD/kg (a moderate wholesale price of industrial grade HAp), we estimated a median annual profit of 1.4 (0.2–2.4) million USD. Incorporating HAp synthesis into existing N recovery technologies can make both N and P recovery from source-separated urine financially viable. Compared to conventional methods (e.g., solid-state, chemical precipitation, sol-gel, hydrothermal)55, HAp synthesis through the osteoyeast platform requires fewer exogenous chemical inputs by fully utilizing the existing phosphorus and urea in fresh urine. Further, the osteoyeast-facilitated process features milder reaction conditions and exhibits high HAp selectivity that is relatively insensitive to variations in reaction conditions, making it suitable for distributed applications at various scales.
Discussion
Urine recycling is an important concept for the development of a sustainable economy. This process can produce a large amount of fertilizers for agricultural applications and significantly reduce the costs and environmental footprints associated with wastewater treatment. However, the utility of this process has been limited due to a lack of economic incentives. To make the process more attractive, we have demonstrated the development of the osteoyeast platform, utilizing urine as a feedstock for the production of high-value chemical hydroxyapatite (HAp).
The TEA suggests that the cost of HAp production using the osteoyeast platform is highly attractive. Assuming an HAp selling price of 50 USD/kg, the annual profit from HAp synthesis was estimated to range between $19.1 and $138/m3 of urine, depending on the system performance and deployment context. In contrast, nitrogen recovery as ammonium sulfate liquid fertilizer through the UD process was estimated to cost around $12–$33/m3 of urine56. Thus, the profit from HAp production can make the UD process financially attractive. Although this margin may not be guaranteed as the scale increases, reducing the cost of HAp synthesis could significantly expand its applications, including air and water purification, soil treatment16, flame resistance and thermal protection57, plastic replacement, and construction materials.
Given the low MPSP and the high market prices of nano-HAp52,53,54, additional processes could also be employed to improve the HAp product quality (e.g., purity, size, and morphology) and potentially further increase the profit margin of this system despite the additional costs. To fully realize this potential, future research and development efforts should focus on advancing purification techniques, optimizing scale-up processes, and exploring regionally-specific market entrance opportunities to ensure broader adoption and commercial success. Our TEA suggests that total phosphorus (TP) concentration and HAp yield are two of the major cost drivers. TP can be easily increased by adding cheap phosphate to the culture, and will naturally increase with diet shifts from animal to vegetal protein sources8. In contrast, improving HAp titer and yield will likely require further modification of the osteoyeast platform.
To increase HAp production, several genetic modifications can be explored. Urine contains sufficient urea to synthesize 60–90 mM of HAp, whereas our current production is limited to ~4 mM. Optimizing the reaction may involve increasing the Ca and P fluxes into both the cytosol and vacuoles, synthesizing polyphosphate in vacuoles, and breaking down polyphosphate to monophosphate in extracellular vesicles (EVs). Additionally, we can explore accelerating the secretion of ACP EVs. For instance, it is understood that diverse pathways such as the vesicle-containing vacuoles, Golgi apparatus secretory pathways, and ESCRT machinery are involved in EV secretion38,39,58,59. Modulating these pathways could change the number of EVs secreted or their size and morphology. Furthermore, the cell wall is an obstacle to EV secretion. Deleting genes for cell wall biosynthesis or engineering enzymes in EVs that modify cell walls could also improve EV secretion.
In addition to serving as a platform for HAp synthesis via the UD process, the osteoyeast platform holds significant potential for a wide range of applications. While we acknowledge the serendipitous nature of this discovery, a relatively simple modification—expression of ureolytic enzymes—successfully activated molecular mechanisms strikingly similar to those mediating HAp synthesis in osteoblasts. This observation suggests that the molecular machinery underlying HAp synthesis in both systems may have evolved from shared ancestral mechanisms. These ancestral pathways likely provided stress resistance against unexpected cytosolic pH fluctuations or facilitated the detoxification of calcium and other metal ions. Over evolutionary time, these mechanisms appear to have been co-opted for the synthesis of lightweight, durable, and impact-resistant bionanocomposites, such as bone, teeth, and crustacean shells. Studying osteoblasts or crustacean cells involved in HAp synthesis remains a challenge due to their biological complexity. However, the osteoyeast platform introduces an alternative paradigm in biomaterial research, offering a simplified and alternative model system for investigating the synthesis of HAp-based bionanocomposites. Additionally, it provides a versatile platform for producing bionanocomposites with commercial potential, thereby opening opportunities for both fundamental studies and applied research in biomaterials science.
In conclusion, the osteoyeast platform offers a promising approach to transforming urine into a valuable resource for hydroxyapatite (HAp) production, delivering significant economic and environmental benefits. By mimicking molecular mechanisms strikingly similar to those used by osteoblasts for HAp synthesis, this platform underscores the divergent and adaptive evolution of these pathways. It also provides a unique opportunity to study and engineer the molecular mechanisms underlying HAp synthesis across diverse eukaryotic species involved in the formation of bionanocomposites. Further optimization of the genetic and biochemical pathways in the osteoyeast platform could improve its efficiency and broaden its applications, contributing to a more sustainable and economically viable bioeconomy.
Methods
Strains, plasmids, and reagents
Escherichia coli Top10 (Invitrogen) was used as the cloning host. The S. boulardii strain carrying auxotrophic mutations (trp1, his3, ura3) was provided by the Jin lab60, and all S. boulardii strains used in this study are listed in Table 1. Unless otherwise specified in the main text, all chemical reagents were purchased from MilliporeSigma. For genomic integration, the following constructs were generated: DUR3 (fIM52) encoding urea amidolyase, DUR1/2 (fIM54) encoding the urea transporter, both DUR3 and DUR1/2 (fIM58), and VPH1 fused to red fluorescent protein (fIM84). Plasmid maps are shown in Supplementary Fig. 6, and annotated sequences are available in Supplementary Data 7–10. The primers used in the creation of these plasmids can be found in Supplementary Table 1.
Yeast transformation
To integrate the DNA fragment into the S. boulardii genome, yeast transformations were performed using the lithium acetate method61. Briefly, overnight yeast cultures were diluted 1:50 in YPD medium (1% yeast extract, 2% peptone, and 2% glucose) and grown until the optical density at 600 nm (OD600) reached ≥ 0.4. A 50 mL culture was then harvested by centrifugation (3000 × g, 5 min), washed twice with 25 mL of sterile water, and resuspended in 1 mL of water. A 100 µL aliquot of the cell suspension was pelleted in a microcentrifuge tube and resuspended in a transformation mixture containing 240 µL of 50% PEG 3350, 36 µL of 1 M LiAc, 10 µL of 10 mg/mL single-stranded DNA, and 74 µL of linearized DNA and water. The yeast cells were heat-shocked at 42 °C for 45 min, then plated onto yeast synthetic defined (SD) agar medium (Sunrise Science) supplemented with CSM-trp, CSM-his, or CSM-trp-his. Plates were incubated at 37 °C until colonies appeared.
Cultivation conditions for HAp production
Engineered yeast cells were grown overnight in YPD medium, washed with PBS buffer, and inoculated into modified yeast nitrogen base (mYNB) medium consisting of 1.71 g/L Yeast Nitrogen Base without ammonium sulfate (#1500, Sunrise Science), appropriate amino acid supplements, 20 g/L glucose, and 50 mM CaCl2. Urea (20 g/L) and/or additional CaCl2 (50 mM) were optionally added at the start or later during cultivation. Cultures were grown at 37 °C for up to 96 h (as shown in Fig. 3). Crystals and cells were collected by filtration through 2.7 μm filter paper, washed with water, and air-dried at room temperature. In some cases, cell or cell-crystal suspensions were analyzed immediately without filtration.
X-Ray diffraction analysis
The cultures were spin down at 3000 × g for 10 min. Pellets were dried at 80 °C for 48 h prior to analysis. The dried material was analyzed on a Rigaku MiniFlex 6 XRD.
Dry state transmission electron microscopy
Dry pellets containing yeast cells and inorganic materials were dispersed in distilled water and deposited onto lacey carbon 300 mesh copper grids. TEM imaging and selected-area electron diffraction (SAED) were performed at the National Center for Electron Microscopy on an FEI ThemIS TEM equipped with an X-FEG gun and a Ceta2 complementary metal oxide semiconductor (CMOS) camera operating at 300 kV. High-angle annular dark field (HAADF) images were acquired in scanning transmission electron microscopy (STEM) mode at 300 kV with a convergence semi-angle of 11.3 mrad. To determine the compositions of the inorganic materials, energy dispersive X-ray spectroscopy (EDS) was performed using a Bruker SuperX windowless EDS detector, which has a solid angle of 0.7 steradian enabling high count rates with minimal dead time for fast STEM-EDS mapping. STEM-EDS elemental mapping was performed at 300 kV with a 5 to 10 min acquisition time.
Live cell fluorescence microscopy
For long time-lapse fluorescence and bright field microscopy, yeast cells were imaged on sterile glass-bottom dishes. Glass-bottom dishes (35 mm with 14 mm #1.5 glass, Cellvis) were treated with 2 mg/mL of Concanavalin A from JackBean (Sigma Aldrich) in 1x PBS and allowed to incubate for 30 min. We then rinsed the glass-bottom 1x PBS to remove unbound protein. Yeast cells grown overnight were then diluted in YNB media supplemented with 20 g/L urea with or without 50 mM CaCl2 as described above and allowed to bind to the surface.
Bright field and fluorescence imaging were performed on an inverted Zeiss Elyra 7 microscope using a Plan-Apo 63x/1.46 NA oil immersion objective (Zeiss) and a Sapphire 488 nm (0.5 W), 561 nm (0.5 W) and a Lasos 642 nm (550 mW) laser, an MBS 405/488/561/641 and EF LBF 405/488/561/641 filter cube followed by a LP 560 or a BP 570-620+LP 655 beam splitter. Calcein and mCherry fluorophores were excited with the 488 nm and 561 nm laser lines, respectively. Brightfield images were taken using a transmission filter cube. The fluorescence image was split on a Duolink adapter using the appropriate beamsplitter and imaged on 2 pco.edge 4.2 high-speed scientific CMOS cameras. Images were then analyzed using Zeiss Zen Black, ImageJ, or FIJI software.
Correlative optical and electron microscopy
A 3 mm Au TEM finder grid (Ted Pella) was plasma cleaned in Ar for 20 s then affixed to a glass-bottom dish using ultra-thin (0.0015 in) polyimide tape. The entire dish with the TEM grid was submerged with a 2 mg/mL solution of Concanavalin A for 30 min at 37 °C. We then washed the dish with 1x PBS and transferred 1 µL of overnight yeast cell culture onto the petri dish. The dish with the cell culture was incubated for another 30 min at 37 °C before the cell media was aspirated off and replaced with mYNB (crystal growth) media (see cultivation conditions for HAp production section above). Regions of interest near the alphabet letter markers on the TEM finder grid were selected for live cell imaging. The images are collected in a fashion similar to the one outlined in the previous section, using a Plan-Neofluar 40x/1.3 DIC WD = 0.21 M27 objective to image a wider field of view. At the end of the fluorescent imaging analysis, the media was aspirated and the dish and 1 µL of PBS were gently deposited away from the TEM grids to avoid any excessive turbulent flow while rinsing away the media. After being rinsed three times, the polyimide tape was gently removed using sharp tweezers, and the TEM grids were allowed to dry in air. After the TEM grids were fully dry, they were loaded into a FEI ThemIS TEM, and the regions of interest identified during the optical microscopy experiments were imaged using the protocols described in the Dry State Transmission Electron Microscopy section.
HAp production in urine
We tested and optimized the culture conditions of strain SB823 for the production of HAp in human urine (purchased from Innovative Research Inc.), which was adjusted to pH 5.5 using acetate (as shown in Fig. 5). We added varying concentrations of CaCl2 (10–50 mM) and inoculated the strain to a final OD of 0.1 in a total culture volume of 15 mL. HAp production was carried out for 5 days, after which we measured the calcium concentration in the supernatant using calcium assay kit (Abcam) and estimated calcium consumption.
After optimizing the conditions, we tested the ability of SB823 and its control strain SB760 to produce HAp. Both strains were inoculated into 15 mL of human urine supplemented with 30 mM CaCl2, adjusted to pH 5.5 using acetate. We collected 50 µL of samples daily and measured the calcium concentration. After 10 days of cultivation, the samples were collected by centrifugation at 3000 × g for 15 min. The resulting precipitate was washed with deionized water and centrifuged again at 3000 × g for 15 min. The pellets were then dried at 80 °C for 48 h prior to dry-weight measurement.
TEA of HAp synthesis
We assumed each location had two fed-batch reactors in parallel for continuous HAp synthesis. Similarly, we assumed two fed-batch fermenters in parallel for osteoyeast cultivation at the central facility. These reactors are equipped with a cleaning-in-place system, agitators, recirculation pumps, and heat exchangers for automatic operation. HAp reactors and equipment were sized based on the urine flow rate and the design batch time62. Osteoyeast fermenter and equipment were sized based on the system-wide demand for yeast biomass, and the cultivation was assumed to resemble a typical industrial production process of S. boulardii63. Blowers are included and sized based on aeration duty for osteoyeast cultivation. Fresh osteoyeast cells were separated from the liquid mixture using centrifuge without further drying. Precipitates collected from the HAp reactors were assumed to be dried with a recessed plate filter press and then incinerated. Total chamber volume of the filter press was estimated based on the moisture content and flowrate of the precipitates64. Fuel input for incineration was estimated based on the higher heating value and dry solid loading rate of the dried precipitates64. Purchase costs (Cp) of the reactors, the centrifuge, the filter press, the incinerator, and all equipment were estimated as power functions of their sizing factor (S) as shown in Eq. 1. Baseline parameter values (i.e., a, b, and c) of these power functions as well as their ranges of uncertainty were calibrated with supplier price data (Supplementary Table 2 and 3).
$${C}_{p}=a\cdot {S}^{b}+c$$
(1)
We considered three major inputs (i.e., source-separated fresh urine (Supplementary Table 4), CaCl2 powder, and osteoyeast inoculum) and two main products (i.e., precipitates and supernatants) for the HAp synthesis reactors. The mass balance of the HAp reactors can be described by Eqs. 2–6, where Mi,j indicates the average mass flowrate of component i in the input or output stream j at a deployed location:
$${M}_{{CaC}{l}_{2},{in}}={M}_{P,{urine}}\times 5.9721533$$
(2)
$${M}_{P,{urine}}={C}_{P,{urine}}\cdot {Q}_{{urine}}\cdot p/n$$
(3)
where 5.9721533 was derived from the stoichiometry of the HAp precipitation process, Cp,urine indicates the average TP concentration of fresh urine, Qurine indicates the average urination rate, and p and n represent the total population served and the number of deployed locations across the city, respectively.
$${M}_{{HAp},{precipitate}}={M}_{P,{urine}}\cdot {f}_{{HAp}}/0.184987$$
(4)
where fHAp is the specified percentage of the theoretical maximum yield, and 0.184987 is the phosphorus content of HAp.
$${M}_{{biomass},{precipitate}}={M}_{{yeast},{inoculum}}+{M}_{{COD},{urine}}\cdot {y}_{{biomass}}$$
(5)
$${M}_{{yeast},{inoculum}}={Q}_{{urine}}\cdot {C}_{{inoculum}}$$
(6)
where Cinoculum indicates the design inoculum concentration in the HAp reactors, ybiomass indicates the overall yeast biomass yield (g biomass/g urine COD) due to yeast growth. ybiomass was calibrated against a yeast-to-HAp mass ratio in the precipitate of 0.34 in the baseline scenario (i.e., fHAp = 66% and Cinoculum = 0.5 g/L), which is representative of the experimental result (Fig. 5d).
Sulfuric acid was dosed to lower the pH of fresh urine to inhibit other microbial activities, urea degradation, and the formation of other precipitates during the HAp synthesis process. We assumed a one-time dosage of 60 meq H2SO4/L fresh urine for a conservative estimation of the corresponding operational cost65.
The mixed influent of source-separated fresh urine, osteoyeast inoculum, and CaCl2 was assumed to have cooled down to 30 °C when it reaches the HAp reactor. A heat exchanger is included for each HAp reactor to maintain a reaction temperature of 37 °C. The heat utility required for continuous operation of the HAp reactors was estimated using a previously defined algorithm for similar fed-batch fermenters62, where the heating duty is calculated as the difference in total enthalpy flows between reactor outputs and reactor inputs (accounting for differences in temperature and in composition). No subsequent cooling is needed for the HAp reactors.
For the osteoyeast cultivation reactors, the feedstocks include organic carbon (in forms of molasses and/or glucose), water, macronutrients (in forms of ammonium sulfate and phosphoric acid), and micronutrients (including minerals and vitamins), besides seed osteoyeast. The output fermentation broth contains significant amounts of osteoyeast cells, ethanol, and water. The mass balance is described by the Eqs. 7–9:
$${M}_{{yeast},{broth}}=n\cdot {M}_{{yeast},{inoculum}}/{f}_{{viable}}$$
(7)
Where fviable indicates the average fraction of viable cells63.
$${M}_{{sugar},{feed}}={M}_{{yeast},{broth}}/{y}_{{yeast}}$$
(8)
$${Q}_{{broth}}={M}_{{sugar},{feed}}/{C}_{{sugar}}$$
(9)
where the feedstock flowrate Msugar,feed is determined by the required production rate of osteoyeast cells Myeast,broth, and the specified fermentation yield yyeast (kg yeast/kg sugar), and the average volumetric flowrate of the fermentation broth Qbroth (m3/hr) is dependent on the design sugar concentration Csugar (kg/m3). The aeration duty of an osteoyeast fermenter is calculated using Eq. 10.
$${Q}_{{air}}={q}_{{duty}}\cdot {V}_{{broth}}$$
(10)
where qduty indicates the amount of air supply required per unit volume of fermentation broth (m3·(hr·m3 broth)−1), and Vbroth is the volume of broth in the fermenter at its full capacity. Vbroth is dependent on the fermenter volume, which is determined by the design retention time, the average feedstock flowrate, and the number of fed-batch reactors in parallel62. Key parameter values for the osteoyeast fermenters can be found in Supplementary Table 5. All feedstocks were assumed to be at 20 °C when fed to the fermenters. Heat exchangers are installed and operated to maintain a fermentation temperature of 35 °C. Heat utility was estimated using the same algorithm as that for the HAp reactors62.
Precipitates from the HAp reactors are collected and then processed at the central facility, first dried mechanically and then incinerated with additional fuel. The recessed plate filter press is sized based on the total flow and moisture content of the precipitates collected, following an established algorithm64. The incinerator is sized to accommodate the required burn rate, which is determined by the average output mass flow rate of the dryer. The final product is assumed to be a powder composed of HAp and ash.
$${M}_{{ash},{product}}={M}_{{biomass},{precipitate}}\cdot {f}_{{inert}}$$
(11)
where finert represents the inert fraction of biomass. finert was fixed at 0.128, a value higher than the ash content of yeast cells to account for other impurities66,67,68. Detailed design and TEA results can be found in the Supplementary Fig. 7 and Supplementary Data 1–6.
To realistically evaluate the costs associated with material distribution and precipitate collection, we assumed the locations for HAp reactor deployments were randomly distributed within a rectangular area that spanned ~8.3 miles longitudinally and 7.0 miles latitudinally, which resembles the size of San Francisco. The location for the central facility was also randomly sampled in this area. Then a capacitated vehicle routing (CVR) problem was formulated, given service time required per location and vehicle capacity as constraints. We used Google OR-Tools to solve the CVR problem69, and the optimized routes for distribution and collection were input to the calculation of traveling distances and time for costing.
For TEA, we assumed a 10-year project lifetime and a 5% discount rate. CAPEX includes installed costs of all unit operations and equipment. All installed costs were converted into 2023 USD using the Chemical Engineering Plant Cost Index70. OPEX includes operation and maintenance labor costs (e.g., fermenter operators, vehicle drivers), electricity and fuel input, material costs (e.g., feedstock for yeast cultivation, CaCl2 for HAp synthesis), and others (e.g., central facility rent, vehicle rental costs). All code for design, costing, and simulation of this system are made available in the EXPOsan Python repository71. TEA was performed using the TEA module in the QSDsan Python package46,72. Parameters varied in Monte Carlo simulations are detailed in Supplementary Table 2.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data supporting the findings of this work are available within the paper and its Supplementary Information files. A reporting summary for this Article is available as a Supplementary Information file. Source data are provided with this paper.
References
Wald, C. The urine revolution: how recycling pee could help to save the world. Nature 602, 202–206 (2022).
Einhorn, C. Meet the peecyclers. Their idea to help farmers is No. 1. The New York Times (2022).
Rich Earth Institute. https://richearthinstitute.org/urine-diversion-guide/.
Larsen, T. A. & Gujer, W. Separate management of anthropogenic nutrient solutions (human urine). Water Sci. Technol. 34, 87–94 (1996).
Larsen, T. A., Alder, A. C., Eggen, R. I. L., Maurer, M. & Lienert, J. Source separation: will we see a paradigm shift in wastewater handling? Environ. Sci. Technol. 43, 6121–6125 (2009).
Trimmer, J. T. & Guest, J. S. Recirculation of human-derived nutrients from cities to agriculture across six continents. Nat. Sustain. 1, 427–435 (2018).
Mihelcic, J. R., Fry, L. M. & Shaw, R. Global potential of phosphorus recovery from human urine and feces. Chemosphere 84, 832–839 (2011).
Trimmer, J. T., Cusick, R. D. & Guest, J. S. Amplifying progress toward multiple development goals through resource recovery from sanitation. Environ. Sci. Technol. 51, 10765–10776 (2017).
Trimmer, J. T., Margenot, A. J., Cusick, R. D. & Guest, J. S. Aligning product chemistry and soil context for agronomic reuse of human-derived resources. Environ. Sci. Technol. 53, 6501–6510 (2019).
Hilton, S. P., Keoleian, G. A., Daigger, G. T., Zhou, B. & Love, N. G. Life cycle assessment of urine diversion and conversion to fertilizer products at the city scale. Environ. Sci. Technol. 55, 593–603 (2021).
Phillips, A. J. et al. Engineered applications of ureolytic biomineralization: a review. Biofouling 29, 715–733 (2013).
Dimitriou, R., Jones, E., McGonagle, D. & Giannoudis, P. V. Bone regeneration: current concepts and future directions. BMC Med. 9, 66 (2011).
Weaver, J. C. et al. The stomatopod dactyl club: a formidable damage-tolerant biological hammer. Science 336, 1275–1280 (2012).
Amini, S., Tadayon, M., Idapalapati, S. & Miserez, A. The role of quasi-plasticity in the extreme contact damage tolerance of the stomatopod dactyl club. Nat. Mater. 14, 943–950 (2015).
Hydroxyapatite Market size, trends and forecast to. https://www.coherentmarketinsights.com/market-insight/hydroxyapatite-market-5636. (2030).
Ibrahim, M., Labaki, M., Giraudon, J.-M. & Lamonier, J.-F. Hydroxyapatite, a multifunctional material for air, water and soil pollution control: a review. J. Hazard. Mater. 383, 121139 (2020).
Chun, K. J., Choi, H. H. & Lee, J. Y. Comparison of mechanical property and role between enamel and dentin in the human teeth. J. Dent. Biomech. 5, 1758736014520809 (2014).
De Belie, N. et al. A review of self-healing concrete for damage management of structures. Adv. Mater. Interfaces 5, 1800074 (2018).
Yeom, B. et al. Abiotic tooth enamel. Nature 543, 95–98 (2017).
Okuda, K., Hirota, K., Mizutani, T. & Numamoto, Y. Enhanced toughness of hydroxyapatite–poly(ethylene terephthalate) composites by immersion in water. Mater. Adv. 2, 5691–5703 (2021).
Abdal-hay, A., Pant, H. R. & Lim, J. K. Super-hydrophilic electrospun nylon-6/hydroxyapatite membrane for bone tissue engineering. Eur. Polym. J. 49, 1314–1321 (2013).
Thomson, R. C., Yaszemski, M. J., Powers, J. M. & Mikos, A. G. Hydroxyapatite fiber reinforced poly(alpha-hydroxy ester) foams for bone regeneration. Biomaterials 19, 1935–1943 (1998).
Ranjan, N., Singh, R. & Ahuja, I. P. S. Investigations for mechanical properties of PLA-HAp-CS based functional prototypes. Mater. Today Proc. 18, 2329–2334 (2019).
Long, F. Building strong bones: molecular regulation of the osteoblast lineage. Nat. Rev. Mol. Cell Biol 13, 27–38 (2011).
Boonrungsiman, S. et al. The role of intracellular calcium phosphate in osteoblast-mediated bone apatite formation. Proc. Natl. Acad. Sci. USA. 109, 14170–14175 (2012).
Lotsari, A., Rajasekharan, A. K., Halvarsson, M. & Andersson, M. Transformation of amorphous calcium phosphate to bone-like apatite. Nat. Commun. 9, 4170 (2018).
Iwayama, T. et al. Osteoblastic lysosome plays a central role in mineralization. Sci. Adv. 5, eaax0672 (2019).
Ballabio, A. & Bonifacino, J. S. Lysosomes as dynamic regulators of cell and organismal homeostasis. Nat. Rev. Mol. Cell Biol. 21, 101–118 (2020).
Holmström, K. M. et al. Signalling properties of inorganic polyphosphate in the mammalian brain. Nat. Commun. 4, 1362 (2013).
Omelon, S. J. & Grynpas, M. D. Relationships between polyphosphate chemistry, biochemistry and apatite biomineralization. Chem. Rev. 108, 4694–4715 (2008).
Hossain, M. N., Afrin, S., Humayun, S., Ahmed, M. M. & Saha, B. K. Identification and growth characterization of a novel strain of Saccharomyces boulardii isolated from soya paste. Front. Nutr. 7, 27 (2020).
Li, S. C. & Kane, P. M. The yeast lysosome-like vacuole: endpoint and crossroads. Biochim. Biophys. Acta 1793, 650–663 (2009).
Hothorn, M. et al. Catalytic core of a membrane-associated eukaryotic polyphosphate polymerase. Science 324, 513–516 (2009).
Papouskova, K., Jiang, L. & Sychrova, H. Vcx1 and ESCRT components regulate intracellular pH homeostasis in the response of yeast cells to calcium stress. FEMS Yeast Res. 15, fov007 (2015).
Miseta, A., Kellermayer, R., Aiello, D. P., Fu, L. & Bedwell, D. M. The vacuolar Ca2+/H+ exchanger Vcx1p/Hum1p tightly controls cytosolic Ca2+ levels in S. cerevisiae. FEBS Lett. 451, 132–136 (1999).
Dunn, T., Gable, K. & Beeler, T. Regulation of cellular Ca2+ by yeast vacuoles. J. Biol. Chem. 269, 7273–7278 (1994).
Rodrigues, M. L. et al. Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot. Cell 7, 58–67 (2008).
Ullah, A. et al. Characteristics and potential clinical applications of the extracellular vesicles of human pathogenic Fungi. BMC Microbiol. 23, 227 (2023).
Zhao, K. et al. Extracellular vesicles secreted by Saccharomyces cerevisiae are involved in cell wall remodelling. Commun. Biol. 2, 305 (2019).
Rizzo, J., Taheraly, A. & Janbon, G. Structure, composition and biological properties of fungal extracellular vesicles. microLife 2, uqab009 (2021).
Sethuraman, A., Rao, N. N. & Kornberg, A. The endopolyphosphatase gene: essential in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 98, 8542–8547 (2001).
Gerasimaitė, R. & Mayer, A. Ppn2, a novel Zn2+-dependent polyphosphatase in the acidocalcisome-like yeast vacuole. J. Cell Sci. 130, 1625–1636 (2017).
Zhu, J. et al. A validated set of fluorescent-protein-based markers for major organelles in yeast (Saccharomyces cerevisiae). MBio 10, e01691–e01619 (2019).
Du, S. J., Frenkel, V., Kindschi, G. & Zohar, Y. Visualizing normal and defective bone development in zebrafish embryos using the fluorescent chromophore calcein. Dev. Biol. 238, 239–246 (2001).
Davies, E. et al. Citrate bridges between mineral platelets in bone. Proc. Natl. Acad. Sci. USA 111, E1354–E1363 (2014).
Li, Y. et al. QSDsan: an integrated platform for quantitative sustainable design of sanitation and resource recovery systems. Environ. Sci. Water Res. Technol. 8, 2289–2303 (2022).
Quantitative Sustainable Design (QSD) Group. QSDsan: Quantitative Sustainable Design (QSD) of Sanitation and Resource Recovery Systems. (Github).
Cortes-Peña, Y., Kumar, D., Singh, V. & Guest, J. S. BioSTEAM: a fast and flexible platform for the design, simulation, and techno-economic analysis of biorefineries under uncertainty. ACS Sustain. Chem. Eng. 8, 3302–3310 (2020).
BioSTEAMDevelopmentGroup. ThermoSTEAM: BioSTEAM’s Premier Thermodynamic Engine. (Github).
BioSTEAMDevelopmentGroup. BioSTEAM: The Biorefinery Simulation and Techno-Economic Analysis Modules. (Github).
Udert, K. M., Larsen, T. A., Biebow, M. & Gujer, W. Urea hydrolysis and precipitation dynamics in a urine-collecting system. Water Res. 37, 2571–2582 (2003).
Hydroxyapatite. https://www.sigmaaldrich.com/US/en/product/sial/21223.
Hydroxyapatite. https://www.sigmaaldrich.com/US/en/product/sial/04238.
Hydroxyapatite. https://www.matexcel.com/hydroxyapatite.html.
Chetty, A., Du Preez, I., Marei, M. K. & Moussa, R. M. Hydroxyapatite: Synthesis, Properties, and Applications 91–132 (Nova, 2012).
Kavvada, O., Tarpeh, W. A., Horvath, A. & Nelson, K. L. Life-cycle cost and environmental assessment of decentralized nitrogen recovery using ion exchange from source-separated urine through spatial modeling. Environ. Sci. Technol. 51, 12061–12071 (2017).
Zhang, Q. et al. Flame-retardant and thermal-protective polyimide-hydroxyapatite aerogel fiber-based composite textile for firefighting clothing. Composites Part B 248, 110377 (2023).
Wollert, T., Wunder, C., Lippincott-Schwartz, J. & Hurley, J. H. Membrane scission by the ESCRT-III complex. Nature 458, 172–177 (2009).
Pfitzner, A.-K. et al. An ESCRT-III polymerization sequence drives membrane deformation and fission. Cell 182, 1140–1155.e18 (2020).
Liu, J.-J. et al. Metabolic engineering of probiotic Saccharomyces boulardii. Appl. Environ. Microbiol. 82, 2280–2287 (2016).
Gietz, R. D. & Schiestl, R. H. High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat. Protoc. 2, 31–34 (2007).
Humbird, D. et al. Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol: Dilute-Acid Pretreatment and Enzymatic Hydrolysis of Corn Stover. https://www.nrel.gov/docs/fy11osti/47764.pdf 10.2172/1013269 (2011)
Zheng, G. et al. Boulardii active dry yeasts and production method thereof. Chinese patent 103,374,531 (2013).
Water Engineering Research Laboratory & Center for Environmental Research Information (U.S.). Handbook: Estimating Sludge Management Costs. (U.S. Environmental Protection Agency, Center for Environmental Research Information, 1985).
Hellström, D., Johansson, E. & Grennberg, K. Storage of human urine: acidification as a method to inhibit decomposition of urea. Ecol. Eng. 12, 253–269 (1999).
Bertolo, A. P., Biz, A. P., Kempka, A. P., Rigo, E. & Cavalheiro, D. Yeast (Saccharomyces cerevisiae): evaluation of cellular disruption processes, chemical composition, functional properties and digestibility. J. Food Sci. Technol. 56, 3697–3706 (2019).
Patterson, R., Rogiewicz, A., Kiarie, E. G. & Slominski, B. A. Yeast derivatives as a source of bioactive components in animal nutrition: a brief review. Front. Vet. Sci. 9, 1067383 (2022).
Lee, H. J., Park, B.-R. & Chewaka, L. S. A comparative study of composition and soluble polysaccharide content between brewer’s spent yeast and cultured yeast cells. Foods 13, 1567 (2024).
OR-Tools. Google for Developers https://developers.google.com/optimization.
The Chemical Engineering Plant Cost Index ®. Chemical Engineering https://www.chemengonline.com/pci-home. (2014).
Quantitative Sustainable Design (QSD) Group. EXPOsan: EXPOsition of Sanitation and Resource Recovery Systems. (GitHub).
Quantitative Sustainable Design (QSD) Group. QSDsan: Quantitative Sustainable Design for Sanitation and Resource Recovery Systems. (Github).
Deptuła, A. et al. Sol-gel-derived hydroxyapatite and its application to sorption of heavy metals. Adv. Sci. Technol. Water Res. 45, 2198–2203 (2006).
Guo, H. et al. Synthesis of a magnetic carnation-like hydroxyapatite/basic calcium carbonate nanocomposite and its adsorption behaviors for lead ions in water. Molecules 27, 5565 (2022).
Acknowledgements
We would like to thank Professor Ro Cusick at University of Illinois Urbana-Champaign for valuable comments to our project. This work was supported by laboratory-directed research and development (LDRD). The work conducted by the U.S. Department of Energy (DOE) Joint Genome Institute (https://ror.org/04xm1d337), a DOE Office of Science User Facility, is supported by the Office of Science of the U.S. Department of Energy operated under Contract No. DE-AC02-05CH11231. Work at the Molecular Foundry was supported by the Office of Science, Office of Basic Energy Sciences, of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231. This research was developed with funding from the Defense Advanced Research Projects Agency (DARPA) as part of the Bio-inspired Restoration of Aged Concrete Edifices (BRACE) program. D.K. would like to acknowledge funding from AFOSR (FA9550-23-1-0209). Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the views of DOE or the US Government. The views, opinions, and/or findings expressed are those of the author and should not be interpreted as representing the official views or policies of the Department of Defense or the US Government. We thank Anita Wahler for professional editing of this manuscript. We acknowledge Eduardo de Ugarte for his artistic contribution to Fig. 1.
Ethics declarations
Competing interests
I.E.M., Y.Y., P.E., and A.Y.W.L. have filed a PCT and US patent application (PCT/US2023/018715) for the osteoyeast platform. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Jens Nielsen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Müller, I.E., Lin, A.Y.W., Otani, Y. et al. Cost-effective urine recycling enabled by a synthetic osteoyeast platform for production of hydroxyapatite. Nat Commun 16, 4216 (2025). https://doi.org/10.1038/s41467-025-59416-8
Received: 02 December 2024
Accepted: 22 April 2025
Published: 06 May 2025
DOI: https://doi.org/10.1038/s41467-025-59416-8
.png)